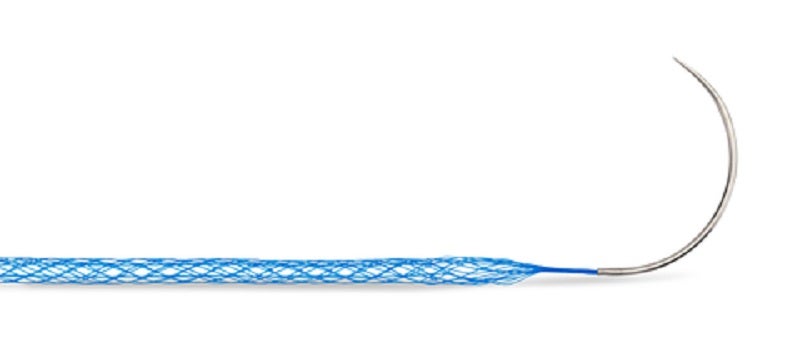
Mesh Suture, which does business as MSi, has secured 510(k) approval from the US Food and Drug Administration (FDA) for Duramesh, a non-absorbable polypropylene mesh suture for the surgical closure of soft tissues, including muscles, fascia, tendons and ligaments.
Duramesh is claimed to be a first-of-its-kind medical device, which uses the implant incorporation in mesh repair in combination with the placement precision of a suture to repair soft tissues.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The device is intended to reduce the problem of surgical failure resulting from suture pull-through.
A regular suture’s sharp leading edge can slice through intact tissue, which can lead to dehiscence, hernia formation and even poor tendon function. To resist pull-through, Duramesh’s architecture flattens right at the suture-tissue interface.
The open-walled hollow design of Duramesh also enables tissue ingrowth for implant incorporation without the formation of a capsule.
Duramesh was found to have fewer hernias and loose sutures numerically when compared to regular sutures in a porcine study.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataMSi chief medical officer Dr Gregory Dumanian said: “Duramesh offers the perfect combination of strength and simplicity in a surgical repair.
“It combines the handling characteristics of traditional suture with a mesh polyfilament design. We are excited to bring this innovative technology to our surgeon colleagues and to their patients who need it most.
“By designing Duramesh to address the surgical complication of suture pull-through, we expect to see sizeable improvements in patient outcomes.”
The device is currently in clinical use in the EU and UK, following receipt of CE Mark designation in Q2 2021.





