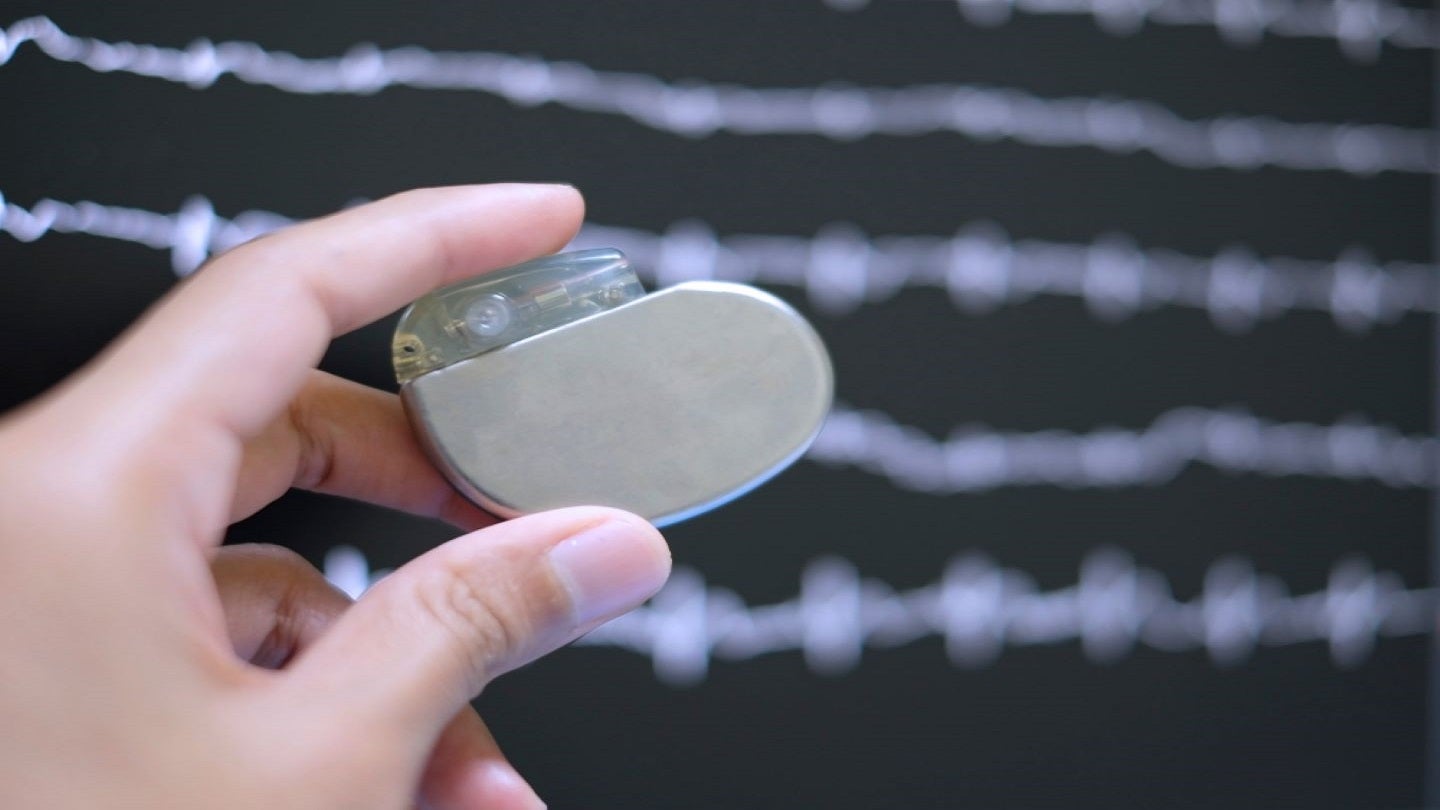
MicroPort CRM has secured approval from the US Food and Drug Administration (FDA) for its next-generation implantable pacemakers, Alizea and Celea.
The regulator has also approved associated products, including Vegapacing leads, the SmartView ConnectBluetooth home monitor and the SmartTouch XTtablet-based programmer.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The AutoMRI mode, an algorithm developed by MicroPort CRM, is integrated into Alizea and Celea. It enhances the safety and quality of life for patients during magnetic resonance imaging (MRI) examinations.
MicroPort CRM president Benoît Clinchamps said: “Alizea and Celeapacemakers, associated with the SmartView Connecthome monitor, are a perfect example of our objective to improve the management of healthcare by reducing hospital visits while ensuring continuity of monitoring and follow-up.”
To activate the MRI mode, patients need to make a single visit to their cardiologist within ten days before their MRI scan.
After the mode is enabled, the pacemaker automatically switches to the MRI mode as soon as it enters the MRI field.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe pacemaker returns to its original setting once the examination is complete, thereby enhancing the workflow for both patients and medical staff.
The Vega pacing leads implanted in these devices enable their safe utilisation within MRI scanners rated at 1.5 and 3 Tesla.
Alizea and Celea are claimed to have the longest lifespan compared to pacemakers of a similar size currently available in the market, with an estimated lifespan of 13 years and a volume of 11cc.
The individuals who receive Alizea or Celea implants are equipped with the SmartView Connect, a home monitor positioned beside their bed.
The interface of the SmartView Connect home monitor provides a user-friendly experience for elderly patients.
The monitor enables cardiologists to automatically and regularly receive comprehensive reports on the system’s performance.
Additionally, it notifies the cardiologist about any irregular heart rhythms such as atrial fibrillation, as well as alerts triggered by the patients when they have symptoms.
Last year, the company also received FDA approval for the EasyFindercardiac electrophysiology diagnostic catheters and PathBuildertransseptal puncture products.





