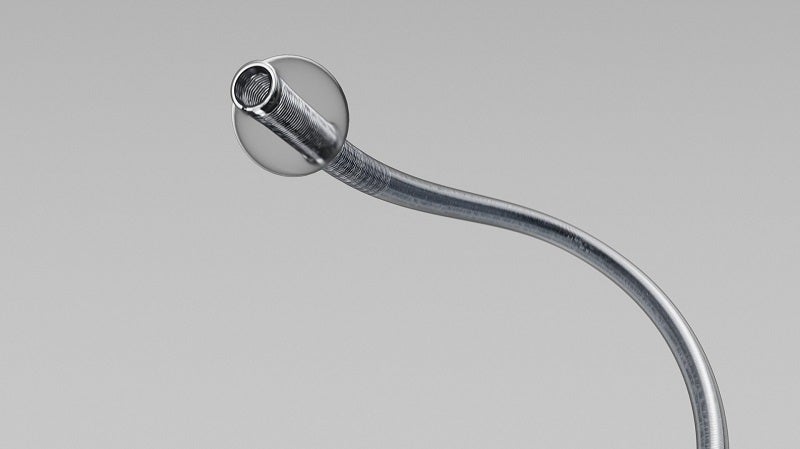
Terumo’s wholly-owned subsidiary MicroVention has announced the enrolment of the first participant in the STRAIT study of its new BOBBY balloon guide catheter.
A multi-centre, prospective observational study, STRAIT is being conducted in the European Union (EU).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It has been designed to assess the BOBBY balloon guide catheter’s safety and performance for endovascular treatment of acute ischemic stroke.
The new balloon guide catheter has been designed for use in enabling insertion and intravascular catheter guidance into a selected blood vessel in the peripheral and neurovascular systems.
During these, and other angiographic procedures, the balloon provides temporary vascular occlusion.
MicroVention president and CEO Carsten Schroeder said: “The STRAIT trial is intended to provide clinical evidence that MicroVention’s new balloon guide catheter can effectively contribute to improved clinical outcomes.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe BOBBY device streamlines the balloon preparation and improves navigability as well as compatibility with the SOFIA Plus 6Fr Aspiration Catheter.
It is currently approved in North America and Europe and is also indicated for use as a conduit for retrieval devices.
The SOFIA Aspiration Catheter is designed for removing/aspiration of emboli and thrombi from selected blood vessels in the arterial system, including the neuro and peripheral vasculatures.
It can be used to facilitate the introduction of diagnostic or therapeutic agents.
Last January, MicroVention’s WEB 17 System, a new addition to the WEB Aneurysm Embolization System, received FDA approval.
Designed with the latest microbraid technology, the WEB 17 System is intended for the treatment of intracranial wide-neck bifurcation aneurysms.





