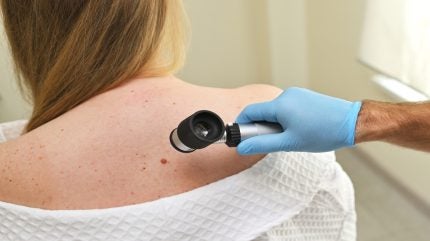
Swedish medical detection company SciBase Holding has seen its artificial intelligence (AI) powered device for detecting melanomas, dubbed Nevisense, validated as part of a consensus report that found it effective in detecting potential cancers.
Published in the Journal of Drugs in Dermatology (JDD), the study saw ten dermatologists reviewing 200 published articles on the topics of non-invasive diagnostic and prognostic testing for cutaneous melanoma (CM) including gene expression profiling (GEP) and electrical impedance spectroscopy (EIS) with the researchers agreeing to a consensus that the combination of methods is able to significantly increase detection.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The research concluded that 2-GEP tests and EIS can aid in the precise diagnosis of clinically indeterminate lesions. According to SciBase, Nevisense is the only US Food and Drug Administration (FDA) approved device for the early detection of skin cancers utilising both systems of treatment.
The device uses EIS to send small electrical signals at different frequencies and signal combinations to the skin and evaluates the response.
Pia Renaudin, CEO of SciBase, said: “I am so excited that this renowned panel of leading dermatologists has endorsed Nevisense as an important tool for early detection of melanoma. This is a significant step forward for SciBase in our US commercialisation process.”
Previously the company had seen success with Nevisense after the California-based Skin and Cancer Reconstructive Surgery Center (SCARS Center) chose to adopt the device in its clinical sites. In 2022, research published in the Journal of Internet Medical Research: Dermatology found that using EIS in the early detection of melanomas is likely to facilitate faster and more reliable biopsy options for clinicians.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAccording to GlobalData’s Pharma Intelligence Center, the number of diagnosed incident cases of melanoma in the eight major markets (UK, US, France, Spain, Italy, Germany, Canada, and Australia) will grow by 16.61% from 190,771 cases in 2019 to 222,458 cases by 2029.
Darrell Rigel, researcher for the consensus study and professor of dermatology at New York University Grossman School of Medicine, said: “Melanoma is one of the top 5 most common cancers in the US and is the main cause of skin cancer deaths in the United States. Early detection is critical to survival and one of the few ways to improve clinical outcomes for patients.
“With melanoma, timing, and the earliest detection possible can make a significant positive impact on survival rates and outcomes. For these reasons, we as clinicians can provide our patients with AI-powered Nevisense, which is an advanced technology that enhances early detection while easily integrating at the point of care.”
Elsewhere in the field of devices designed to detect melanoma, Orlucent has reported positive results from a study evaluating its handheld point-of-care molecular skin fluorescence imaging (mSFI) system for detecting melanoma.





