The UK’s National Institute for Health and Care Excellence (NICE) has recommended AliveCor’s KardiaMobile as an option to detect atrial fibrillation (AF) for suspected paroxysmal AF people.
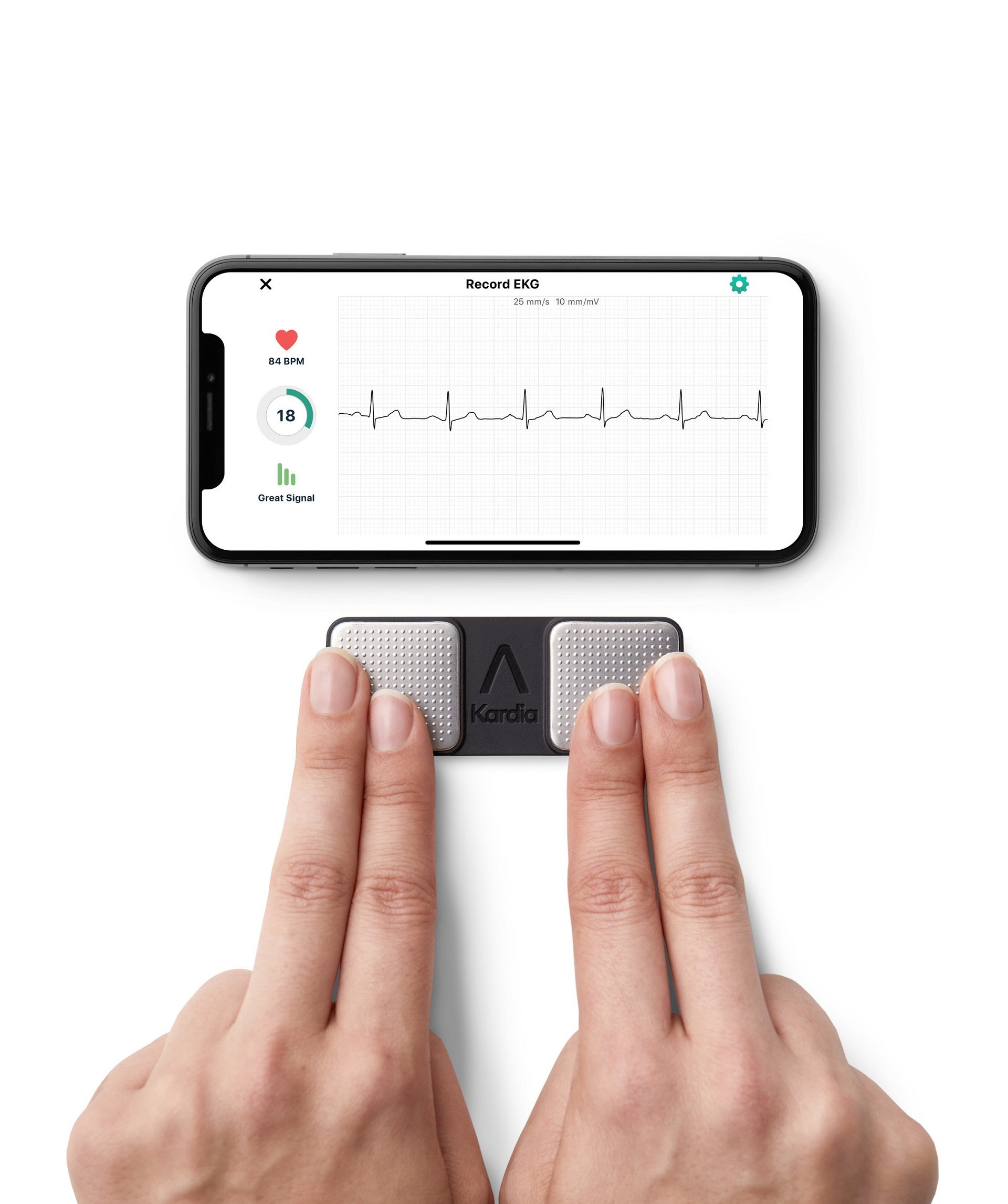
KardiaMobile is a smartphone-based personal electrocardiogram (ECG) solution that can be used to monitor AF at home.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It offers a patchless, wireless and compact solution that can be used anywhere and anytime.
The device is claimed to be the first smartphone-based ECG to be recommended by NICE for use within the National Health Service (NHS).
With this latest approval, physicians and patients across England and Wales will be able to get an ECG recording on their smartphone in 30 seconds with the Kardia app.
AliveCor stated that KardiaMobile provides instant analysis of AF, bradycardia and tachycardia, the leading cardiovascular disease indicators.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataAliveCor CEO Priya Abani said: “AliveCor is proud to be able to offer the only NICE-recommended personal ECG to support remote cardiac care services for patients not in front of their cardiologist.
“Today’s recommendation not only highlights the clinical superiority of KardiaMobile against the current standard of care but also its position as a more cost-effective solution, therefore warranting its value as a clinical tool to support rapid diagnosis of AF.”
The company noted that the latest recommendation is based on evidence from 27 different studies, including five randomised controlled trials.
Findings from these studies and trials showed that KardiaMobile was five times more efficacious at detecting problems related to heartbeat than standard tests.
Last July, AliveCor’s KardiaMobile 6L received 510(k) clearance from the US Food and Drug Administration (FDA) for healthcare experts measuring QTc interval in patients.





