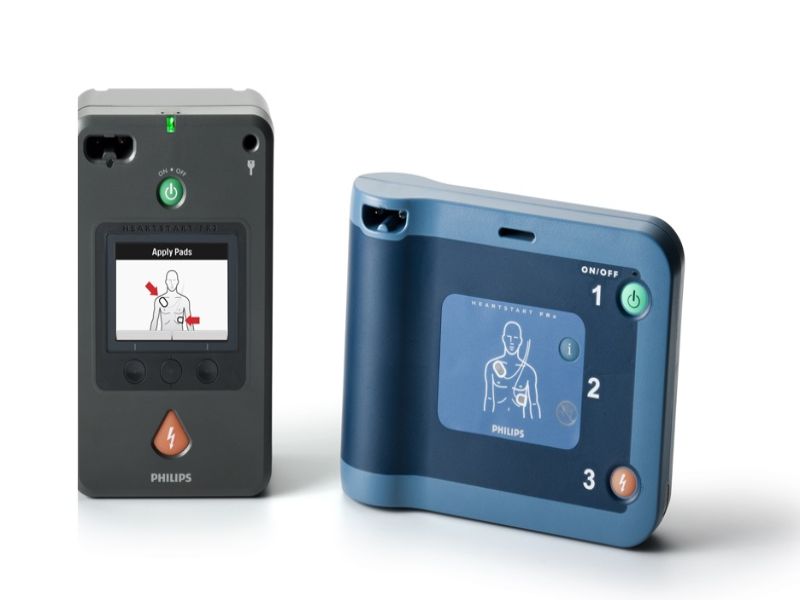
Royal Philips has received premarket approval (PMA) for its HeartStart FR3 and HeartStart FRx automated external defibrillators (AEDs) from the Center for Devices and Radiological Health (CDRH) of the US Food and Drug Administration (FDA).
The HeartStart FR3 is a professional-grade AED for medical personnel and first-responders. It is designed to treat ventricular fibrillation (VF), the most common cause of sudden cardiac arrest (SCA), and pulseless ventricular tachycardias (VTs).

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The HeartStart FRx is a public-access AED for emergency use in workplaces, schools and other public spaces. It has step-by-step voice instructions, including cardiopulmonary resuscitation (CPR) guidance.
The devices had been marketed in the US under 510(k) clearance.
Philips Therapeutic Care business leader Arman Voskerchyan said: “We are pleased to receive premarket approval for our HeartStart FR3 and HeartStart FRx AEDs.
“Our industry-leading portfolio of AEDs is instrumental in helping save the lives of numerous sudden cardiac arrest victims in the US and worldwide. We look forward to continuing to meet our commitment to our medical professional and public-access customers, and especially to the victims of sudden cardiac arrest who rely on our AEDs.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLast year, Philips received PMA approval for its HeartStart OnSite and HeartStart Home defibrillators.
Earlier this month, the company obtained clearance from Japan’s healthcare authority to launch its Lumify with Reacts handheld tele-ultrasound solution in the country.
Philips received 510(k) clearance from the US FDA last month for its wearable biosensor to monitor Covid-19 patients in hospitals.
The company has a range of ultrasound solutions are approved for the management of Covid-19-related lung and cardiac complications in markets such as Brazil, Australia, Canada, the EU, New Zealand and the US.





