PQ Bypass has obtained conditional approval from the US Food and Drug Administration (FDA) to conduct a pivotal clinical trial of its DETOUR System under an investigational device exemption (IDE).
The prospective, single-arm, global multi-centre DETOUR II trial will evaluate the safety and effectiveness of the device to perform percutaneous femoropopliteal bypass called DETOUR procedure.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Set to be conducted at around 40 clinical sites, the trial will involve approximately 292 subjects suffering from lower limb ischemia in the superficial femoral artery (SFA) due to peripheral artery disease (PAD).
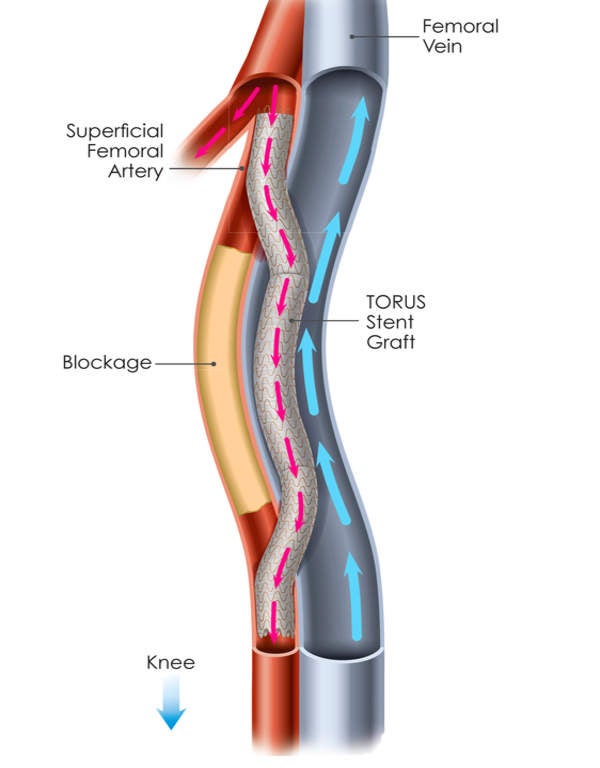
The DETOUR System, which comprises TORUS Stent Graft, DETOUR Crossing Device and DETOUR Snare, will be used during the DETOUR procedure to create a pathway that begins in SFA, passes through the femoral vein and ends in popliteal artery, bypassing the artery’s diseased part.
The trial aims to support a pre-market approval (PMA) submission to the FDA for the DETOUR System.
DETOUR II national co-principal investigator Sean Lyden said: “The DETOUR procedure is designed to treat patients with severely calcified or long-segment disease.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalData“It’s essentially a femoropopliteal bypass with polytetrafluoroethylene, but done percutaneously.
“The DETOUR I trial in Europe demonstrated safety and efficacy in patients with lesions as long as 44cm in length, and we look forward to continuing to study this procedure with the commencement of DETOUR II.”
DETOUR II will also include an economic study to obtain cost-related data associated with PAD treatment in the study population.
The collection of quality-of-life outcome measures, procedural and follow-up costs such as rehospitalisations through 24 months will be carried out by an economics core lab.





