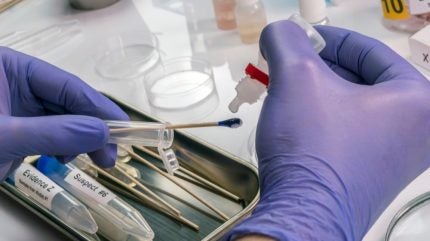
The US Federal Bureau of Investigation (FBI) is partnering with Netherland-based Qiagen to develop a novel assay capable of detecting and quantifying minimal amounts of DNA with high accuracy.
The first-of-its-kind digital polymerase chain reaction (PCR) is expected to boost forensic DNA evaluation to create a better DNA profile.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Forensic samples usually contain a small amount of DNA, PCR can be useful for expanding the sample for evaluation. Additionally, the DNA sample can be damaged by age or environmental factors such as soil. Digital PCR has a higher tolerance to these inhibitors compared to traditional PCR and allows for higher accuracy, according to Qiagen.
Qiagen has previously worked with the FBI, on next-generation sequencing (NGS) technology, ForenSeq MainstAY workflow, to improve workflow. The technology allows for standardised workflow while allowing for more samples to be evaluated, up to 96 samples per run. Qiagen added ForenSeq MainstAY workflow to its portfolio as part of the $150m acquisition of Verogen, in January 2023.
The new digital PCR assay will be run on Qiagen’s QIAcuity digital PCR devices. The QIAcuity platform is an automated nanoplate-based system that counts the presence or absence of DNA molecules. It generates results in approximately two hours.
The market for PCR systems is expected to grow from being worth approximately $700m in 2023 to over $1.2bn in 2033, as per GlobalData market analysis. The digital PCR system segment is expected to see growth of over $300m, with the market growing from being worth $480m in 2023 to $815m in 2033.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataApart from forensics, PCR systems are commonly used as an in vitro diagnostic for the detection of different viruses. In February, Autonomous Medical Devices Incorporated (AMDI) received a National Institutes of Health (NIH) Rapid Acceleration of Diagnostics (RADx) Tech award of up to $5.3m in financing to develop a fast PCR system. The system is expected to detect up to 32 targets per sample in less than ten minutes.





