Reflow Medical has received breakthrough device designation for its Temporary Spur Stent System from the US Food and Drug Administration (FDA).
The retrievable stent technology is intended to treat below-the-knee (BTK) peripheral artery disease.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
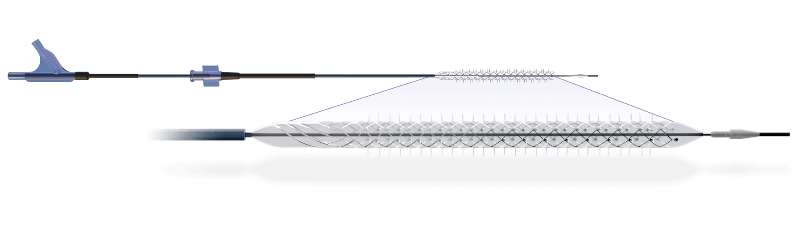
The Temporary Spur Stent System comprises a patented retrievable stent system. It features a series of radially expandable spikes to create numerous pathways.
These pathways will assist in delivering antiproliferative drugs and ensure increased uptake into the vessel wall and also facilitate acute luminal gain, the company claims.
Reflow Medical CEO Isa Rizk said: “We are extremely grateful to the FDA for their expedited designation of the Temporary Spur Stent System as a Breakthrough Device.
“We plan to take full advantage of the Program’s benefits, accelerating our efforts towards meeting the requirements of the review process as we advance this novel technology, with the goal of improving the lives of patients.”

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe breakthrough devices programme aims towards offering patients and health care providers timely access to medical devices that give effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions.
Currently, the Temporary Spur Stent System is not approved for sale and can be used only for an investigational purpose.
The company, in its press statement, said that based on the Spur technology platform it intends to develop in other clinical areas.
Based in California, Reflow develops various products to treat cardiovascular disease.



