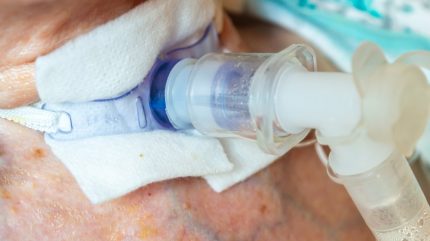
A week after recalling its infusion pumps, Smiths Medical, part of ICU Medical, has sent out an urgent medical device notification regarding “potential issues” with some of its Bivona tracheostomy tubes.
The issue is due to a manufacturing defect that may result in a torn or broken flange, which could lead to displacement or decannulation of the tracheostomy tube. This can result in an “inability to properly ventilate or protect the airway and may contribute to a catastrophic adverse event”.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
A tracheostomy is a surgical procedure where an opening is created in the trachea (windpipe) and a tracheostomy tube is inserted. This is done to provide an air passage to help a patient breathe when the usual route for breathing is somehow blocked or reduced. The procedure is often done when patients are put on ventilators.
The letter was sent on 29 May 2024 to all customers who received any affected product. Smiths Medical stated that it will provide customers with a replacement and/or credit.
The letter went on to outline the required customer actions. Smiths Medical noted that customers should check their inventory against the affected catalogue numbers list provided by the company. The affected products are not used and can be either discarded or quarantined until disposal. Smiths Medical has also notified the US Food and Drug Administration (FDA) of the issue.
GlobalData expects the market for tracheostomy tubes to steadily increase both in the total value and volume from 2024 through to 2033. The market is expected to be worth approximately $182.2m in 2024, to over $206.5m by 2033.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataSmiths Medical has had two device recalls this year. In April, the company issued a Class I recall of its transport ventilators. The recall followed 177 reports of device issues and eight injuries related to the Pneupac Parapac Plus ventilator kits.
In August, Smiths Medical issued a device correction for its CADD-Solis and CADD-Solis VIP infusion pumps after software issues. The recall affected devices with older software versions and can result in multiple operational failures, including malfunctioning alarms and detection systems.



