A day after hitting its paediatric enrolment goal, Spectral AI has widened the reach of its pivotal burn study by adding two clinical trial sites in the US.
The addition of the University of California San Diego and the University of Utah brings the total number of US clinical trial sites to 14.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
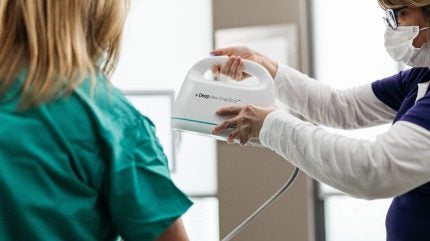
The pivotal study (NCT06131203) is evaluating the US company’s AI wound diagnostics platform DeepView.
Spectral AI’s CEO Peter Carlson said: “These two new sites allow us to capture additional burn patients who enter through the emergency department and thus complement our existing trial site presence at burn centres across the country, establish our presence in new geographies, and advance us towards our objective of completing this study by the end of the year.”
Carlson added that an expanded base of clinical trial sites provides access to a more diverse patient population and reduces bias. This is especially important in imaging devices as skin tone and patient anatomy can affect results.
DeepView uses algorithms to analyse multispectral imaging of wounds against a burn biopsy tissue database. Spectral AI says the device can help predict wound healing outcomes and support clinical decision-making. Assessing the deepness and severity of a burn early is critical for patients to ensure appropriate care.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThe wound care management market is expected to total $38.8bn by 2030, with a CAGR of 3.4%, according to analysis by GlobalData.
Alongside spectral imaging, laser, optical camera, and photoacoustic imaging techniques are used to assess burn depth.
Formerly known as Spectral MD, Spectral AI changed its name after a business combination with special purpose acquisition company (SPAC) Rosecliff Acquisition Corp I. The company’s technology, which received approval for use in adults in the UK in February 2024, is being evaluated in the trial as it looks ahead to US Food and Drug Administration (FDA) approval.
Spectral AI aims to complete the pivotal trial in Q42024, with approval prospects slated for 2025.
In March 2024, the company secured a contract with the Defense Health Agency (DHA) and the US Army Medical Material Development Activity (USAMMDA). The goal of the funding is to support the development of a handheld version of its system for use by military personnel in the field.





