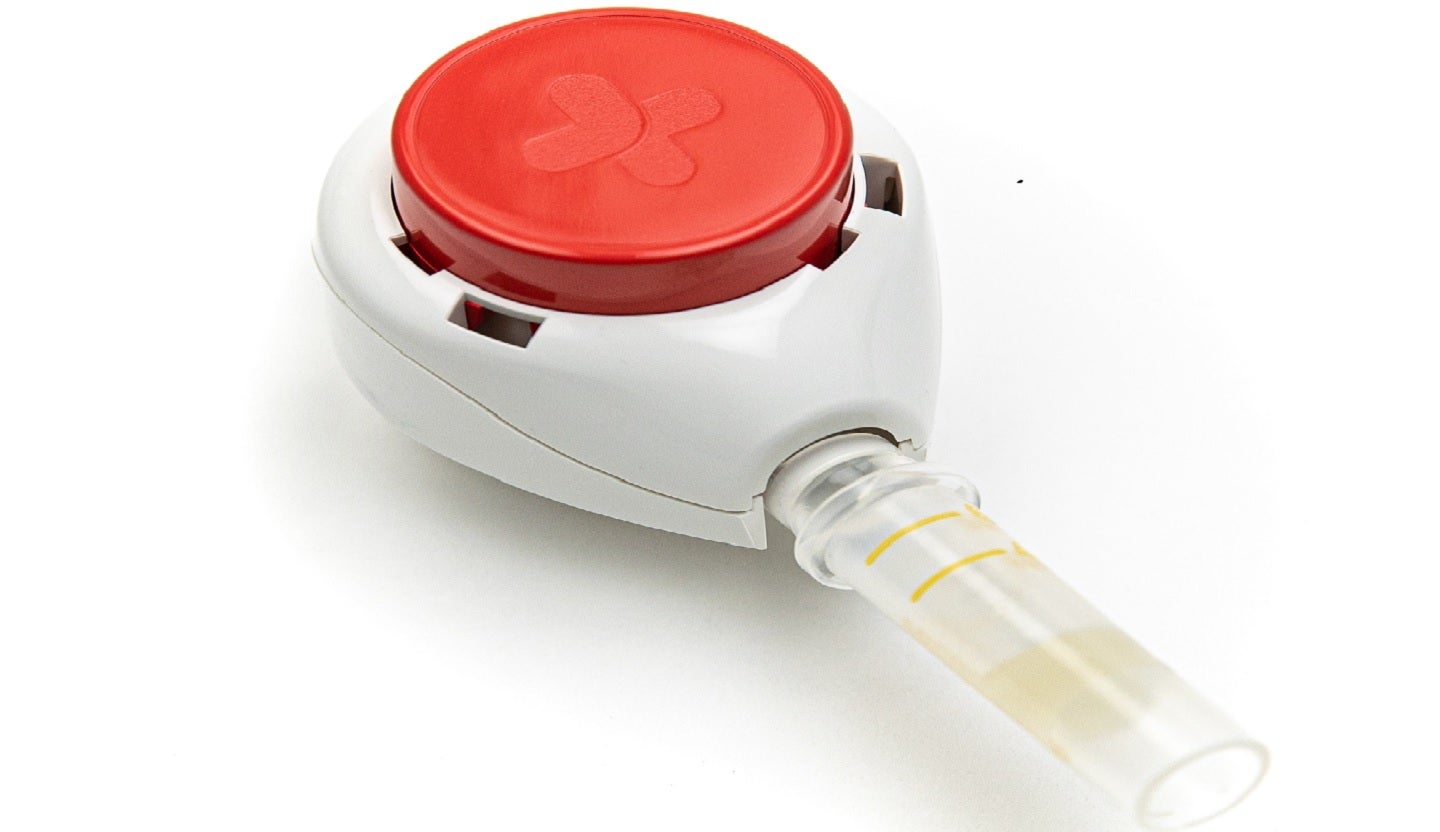
Tasso has received CE mark certification for its new TassoOne Plus high-volume liquid blood collection device.
The company stated that the new device met all safety, performance and relevant product requirements under the new European Union Medical Device Regulation.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
It is said to be the latest addition to Tasso’s portfolio of CE-marked blood collection solutions for dried and liquid samples.
TassoOne Plus allows users to collect their own blood samples at home with an easy and virtually painless process.
Using the new device, this sample collection process offers high volumes and superior sample quality compared to traditional remote blood sampling processes.
The samples collected using TassoOne Plus can be used with the existing downstream analysis workflows of pharmaceutical companies, academic institutions and healthcare organisations in the European Economic Area.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataTasso CEO and co-founder Ben Casavant said: “The demand for convenient, patient-centric care is exploding, and Tasso is on a mission to bring high-quality healthcare into homes worldwide.
“This CE Mark unlocks clinical-grade liquid blood collection for decentralised clinical trials and home healthcare within the European Union, accelerating and expanding access to care. Regulatory clearances like this one are a testament to the quality and safety of our products.”
With the latest CE mark approval, the company will now expand its high-volume, patient-centric blood collection solutions offering for the European market.
Last September, Tasso received clearance from the US Food and Drug Administration (FDA) for its Tasso+ lancet device as a Class II medical device.
The clearance allowed the company to market and sell the device to more healthcare organisations, pharmaceutical companies and academic institutions in the US.



