Visit our Covid-19 microsite for the latest coronavirus news, analysis and updates
Follow the latest updates of the outbreak on our timeline.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
The US Food and Drug Administration (FDA) has granted emergency use authorisation (EUA) to Terumo BCT and Marker Therapeutics for blood purification device to treat acute respiratory failure in Covid-19 patients.
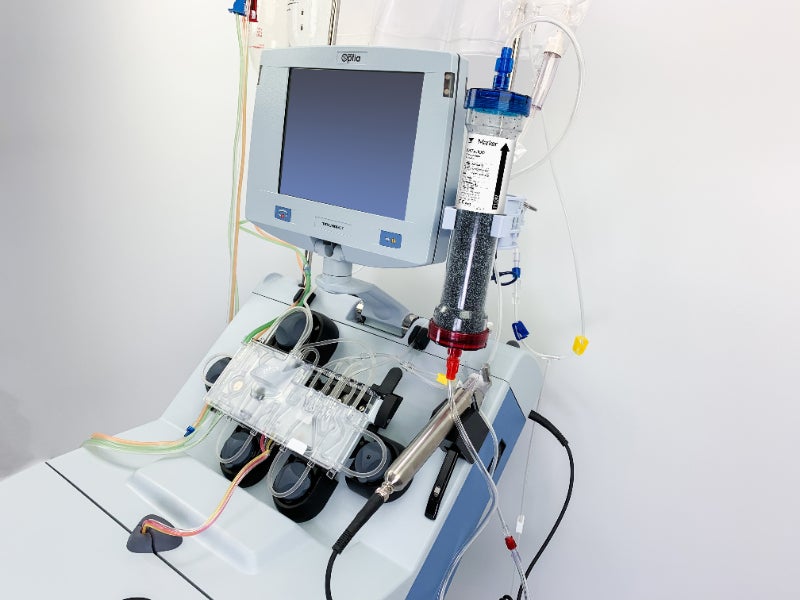
The authorisation has been given for the use of Terumo BCT’s Spectra Optia Apheresis System along with Marker Therapeutics’ Depuro D2000 Adsorption Cartridge.
This combination is indicated to treat Covid-19 patients aged 18 years or older with Covid-19, admitted to the intensive care unit (ICU), with confirmed or imminent respiratory failure.
Together the devices filter the blood and return it to the patient. This reduces the number of cytokines and other inflammatory mediators. Cytokines are active proteins in the bloodstream that control a cell’s immune response.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataThese proteins, which are removed, are normally elevated during infections and are also associated with a ‘cytokine storm’ that occurs in some Covid-19 patients, causing severe inflammation, rapidly progressive shock, respiratory failure, organ failure and death.
Marker Therapeutics chairman David Cohen said: “By combining our plasma adsorption cartridge with Terumo BCT’s technology, this partnership offers the potential to develop a unique global solution for treatment of acute respiratory failure in Covid-19.”
Terumo BCT and Marker Therapeutics collaborated to combine their existing technologies to offer this new approach to potentially treat severe respiratory symptoms caused by Covid-19.
Terumo BCT CEO and president Antoinette Gawin said: “The pace of this collaboration between the companies is incredible. We are leaving no stone unturned in exploring existing and new ways for our products to mitigate the impact of Covid-19.”





