Medical artificial intelligence (AI) solution development company VUNO has received the Class IIa CE markings for five of its AI-based medical solutions.
VUNO Med-BoneAge, VUNO Med-DeepBrain, VUNO Med-Chest X-Ray, VUNO Med-Fundus AI and VUNO Med-LungCT AI are the solutions that have obtained the CE marking.

Discover B2B Marketing That Performs
Combine business intelligence and editorial excellence to reach engaged professionals across 36 leading media platforms.
Following the approval, the company plans to market the products in 27 member states of the EU, the acceding countries and the European Free Trade Association (EFTA) states.
They will also be launched in countries in the Middle East, Asia, South and Central America and Africa where European CE mark is recognised.
The company noted that its Fundus AI, Chest X-ray, BoneAge and VUNO Med Lung CT AI solutions received regulatory approval based on the data from the clinical trials, which demonstrated its efficacy and safety.
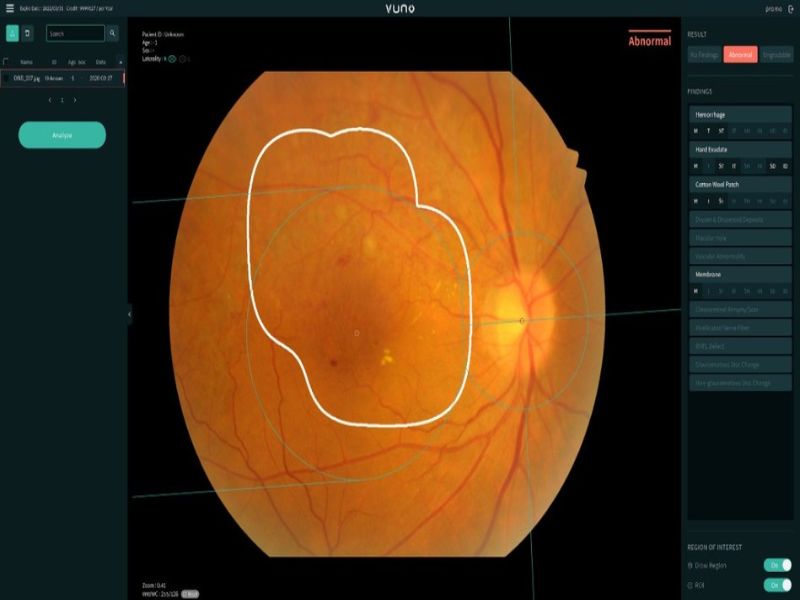
VUNO Med – Fundus AI is developed to automatically detect a range of lesions from the fundus image and classify them for diagnosis.

US Tariffs are shifting - will you react or anticipate?
Don’t let policy changes catch you off guard. Stay proactive with real-time data and expert analysis.
By GlobalDataLung CT AI solution detects and quantifies pulmonary nodules that are likely to progress to lung cancer. It also automatically categorises Lung-RADS to help manage different pulmonary nodules.
The company’s Chest X-Ray solution is designed to detect the most common thoracic findings and diseases through chest X-ray images, while BoneAge helps to evaluate bone age based on a child’s left-hand X-ray image.
The company’s DeepBrain solution segments brain regions, using brain magnetic resonance imaging (MRI) data and quantifies the volumes of each region to offer atrophy volumetrics against the normative database. It also delivers an analysis report.
VUNO CEO Hyun-Jun Kim said: “We have been taking proper measures to ensure that VUNO’s products obtain classification commensurate with their intended purposes as medical diagnostic supporting tools.
“In this sense, obtaining CE certification for all five products holds a great significance, and this will be able to accelerate our push for much anticipated global supply of VUNO Med series all around the world.”
In April, VUNO Med-Fundus AI received Class III medical device approval from the Korean Ministry of Food and Drug Safety.





