ABK Biomedical is set to begin a pivotal clinical trial with its Eye90 microspheres yttrium-90 (Y90) radioembolisation therapy in the US.
The company has secured approval from the US Food and Drug Administration for its investigational device exemption application to start the trial, called Route90.
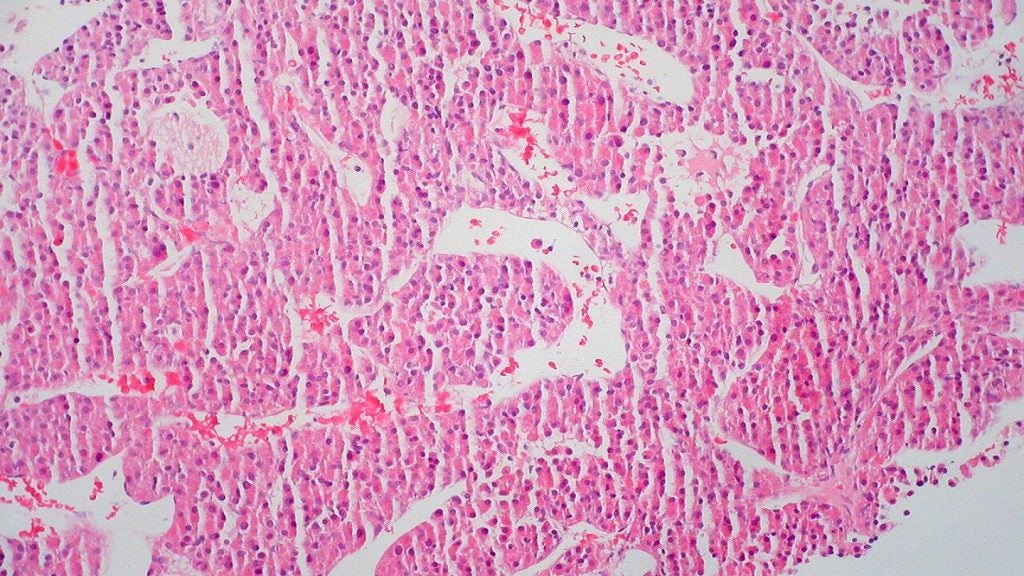
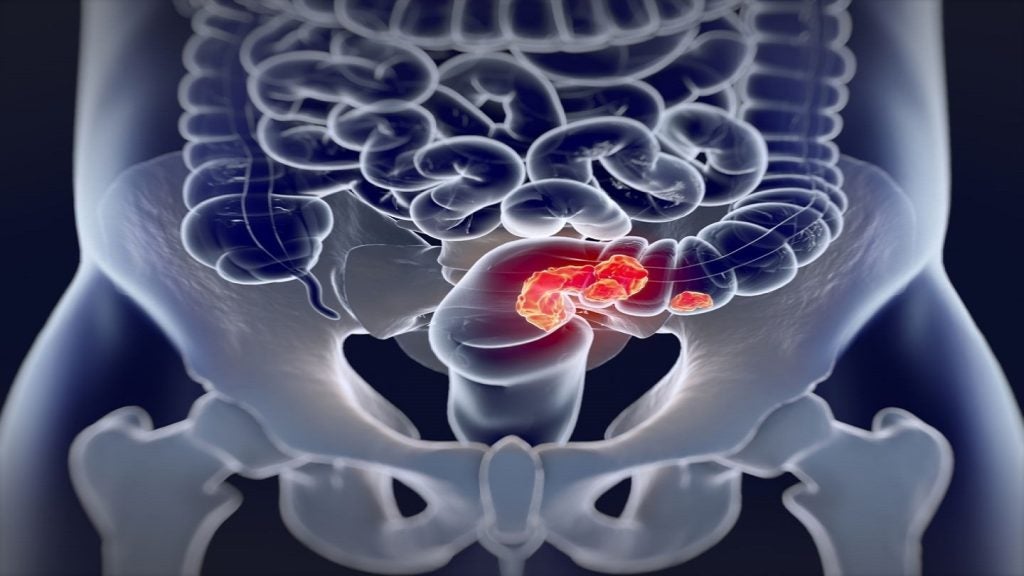
The trial will assess the safety and efficacy of Eye90 microspheres in patients suffering from unresectable hepatocellular carcinoma (HCC).
Eye90 microspheres is claimed to be the first and only imageable Y90 microspheres device.
ABK Biomedical president and CEO Mike Mangano said: “We have created extensive manufacturing and supply-chain efficiencies, established robust quality assurance in all our processes and collaboratively engaged regulatory bodies for proper guidance.
“We believe this will become a seminal study for treating patients with unresectable HCC. We designed our Y90 radio embolisation technology to align with the most recent, advanced Y90 treatment methods, techniques and appropriate patient populations.”
HCC tumours’ response rates and duration of response from treatment with Eye90 microspheres are the co-primary endpoints of the study.
Furthermore, the trial covers endpoints to assess the safety and capability of conducting post-treatment CT-dosimetry with imageable microspheres. It will also explore the potential benefits of intra-procedural visualisation.
Sarah Cannon Cancer Institute’s radiation oncology chief Dr Andrew Kennedy will be the principal investigator of the study, who explained saying: “Eye90 microspheres is a significant and meaningful technology advancement to Y90 radioembolisation therapy not seen in over almost two decades since the current therapies became clinically available.”