Alucent Biomedical has announced plans to commence a clinical trial of its AlucentNVS technology in the US.
The decision comes after the US Food and Drug Administration (FDA) granted an investigational device exemption (IDE) for the study.
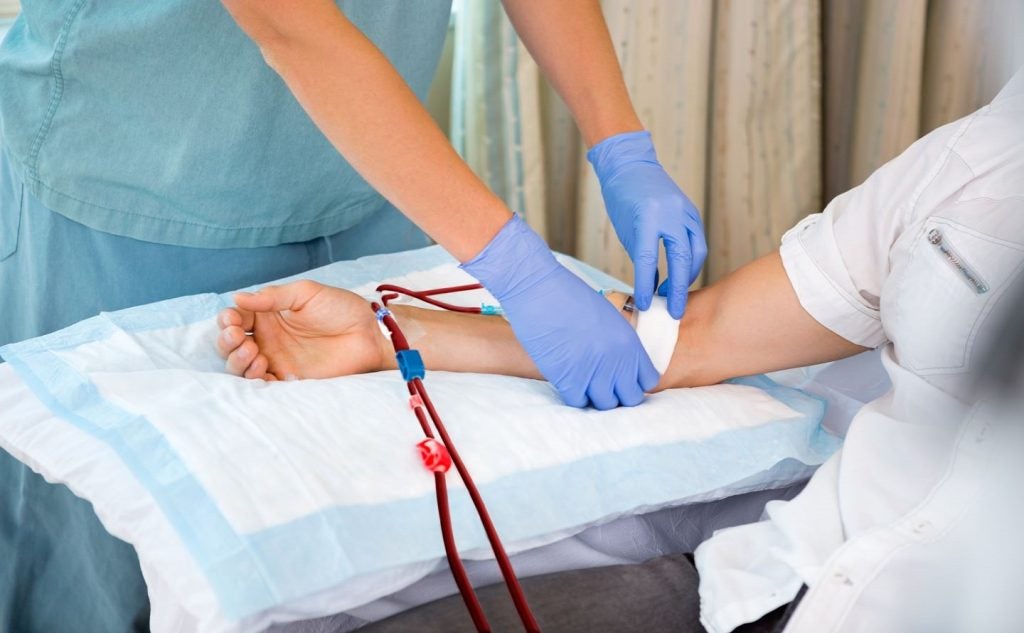
The study will evaluate this technology to promote the maturation of arteriovenous fistulas (AVF) in haemodialysis patients.
AlucentNVS integrates an intravascular device with a photochemical process for connecting structural proteins in a blood vessel wall to control vascular remodelling.
The intervention is intended to promote the patency of the vessel’s lumen and create sustained improvements in blood flow.
The technology helps retain the vascular wall’s natural functionality and flexibility.
Furthermore, the company will leverage its AlucentNVS technology in the surgical AVF creation procedure to enhance the success rate of AV fistula maturation.
Alucent Biomedical CEO Dr Myles Greenberg said: “This second IDE approval by the FDA will allow us to advance our goal to offer physicians and patients a more successful approach to initiating haemodialysis through an AVF in patients with kidney failure, a life-saving procedure where failure rates are still unacceptably high.
“We believe our technology has the potential to transform the current standard of care for patients requiring dialysis.”
The latest development comes after the company received its initial FDA IDE approval in August of this year.
The Activate AVF feasibility trial has already started and is currently enrolling subjects at locations in Poland and Australia.