Amphix Bio has received a breakthrough device designation from the US Food and Drug Administration (FDA) for its drug-device combination product aimed at bone regeneration.
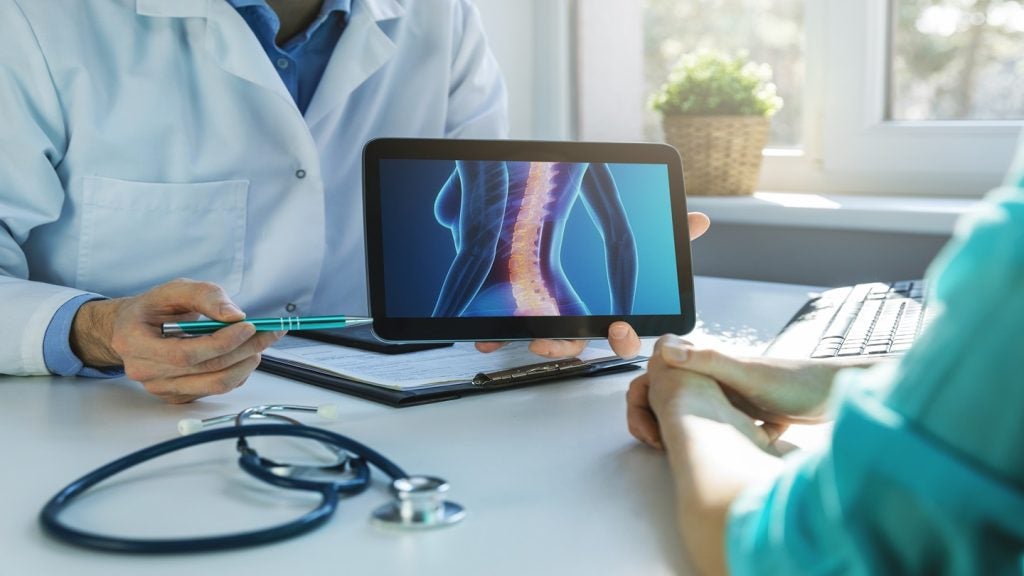
This designation specifically pertains to the treatment of degenerative disc disease through transforaminal lumbar interbody fusion (TLIF) procedures.
Amphix Bio’s product has a mouldable material that allows for easy application by surgeons in various surgical contexts and complex anatomies.
The implant is designed to induce growth of the bone without the need for donor tissue or recombinant proteins. This advancement could present significant benefits over existing products for TLIF spinal surgeries.
It is claimed to be the first time that the FDA is evaluating a product based on supramolecular peptide amphiphiles, which is Amphix Bio's foundational technology platform.
Amphix Bio co-founder and chief scientific officer Samuel Stupp said: “This designation from the FDA is a major milestone for supramolecular therapeutics, and validates the high unmet need that our approach addresses.
“The expedited assessment and review are especially important given that we are aiming to advance an entirely new regenerative medicine platform to the clinic.”
The breakthrough programme aims to expedite the creation, evaluation and review process for medical devices or drug-device hybrids that promise to improve the treatment of life-impairing illnesses.
Amphix Bio is engaged in the development of a new class of regenerative medicine therapies.
The company also provides an off-the-shelf bone graft substitute that enables orthopaedic surgeons to perform spinal fusion surgeries without relying on donor tissue or recombinant proteins, which are commonly used in current methods.