Analog Devices (ADI) has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Sensinel cardiopulmonary management (CPM) system.
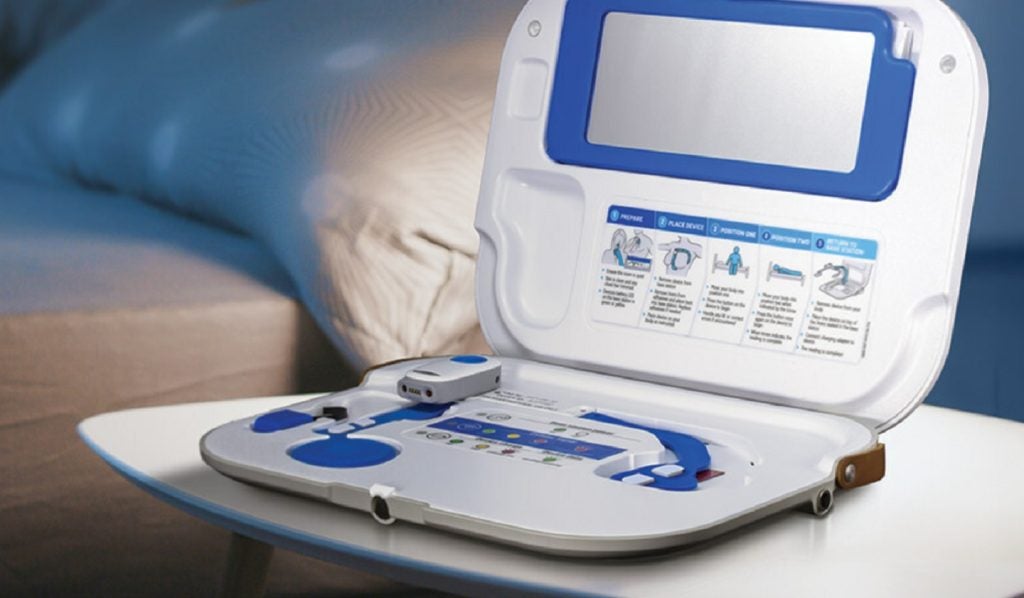
The Sensinel CPM System is a non-invasive, remote management and wearable device. It is designed to capture vital cardiopulmonary measurements for chronic disease management such as heart failure.
ADI Digital Healthcare senior vice-president Patrick O'Doherty said: “By combining our wearable vital signs sensing and signal processing technology with cardiologist-inspired algorithms to precisely determine a congestive heart failure patient's daily state of health, we developed the Sensinel CPM System.
“This innovative service-based product has the potential to open up several billion dollars of green-field market opportunity for ADI while improving patient care, streamlining clinician workflows, and reducing healthcare cost.”
The Sensinel CPM System makes use of a suite of physiological indicators to enable healthcare teams to manage chronic conditions more effectively from a distance.
The wearable device allows patients to self-apply and wear for three to five minutes at home. It can then collect critical health data and transmits it to the Sensinel CPM Cloud Platform through a cellular connection.
ADI said the Sensinel CPM System, which is the inaugural product under the new Sensinel by Analog Devices brand, is now made commercially available.
ADI Medical Products managing director Dr Venu Gopinathan said: “When managing chronic conditions like heart failure, it is critical to adjust treatment early to get the condition under control without the need for hospitalisation. Other existing non-invasive solutions are not specific enough to provide the data a clinician needs to be effective for early intervention.
“Our new cardiopulmonary system is designed to fit seamlessly into the workflow of care and perform a variety of physiological measurements that allow care teams to make early clinical decisions, without subjecting them to information overload.”