Aquedeon Medical has secured investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA) to carry out a study of its Duett Vascular Graft System.
The company will begin the staged pivotal clinical trial of the device in the second half of this year.
For the first stage, the trial will enrol up to 20 patients at up to five clinical sites in the US.
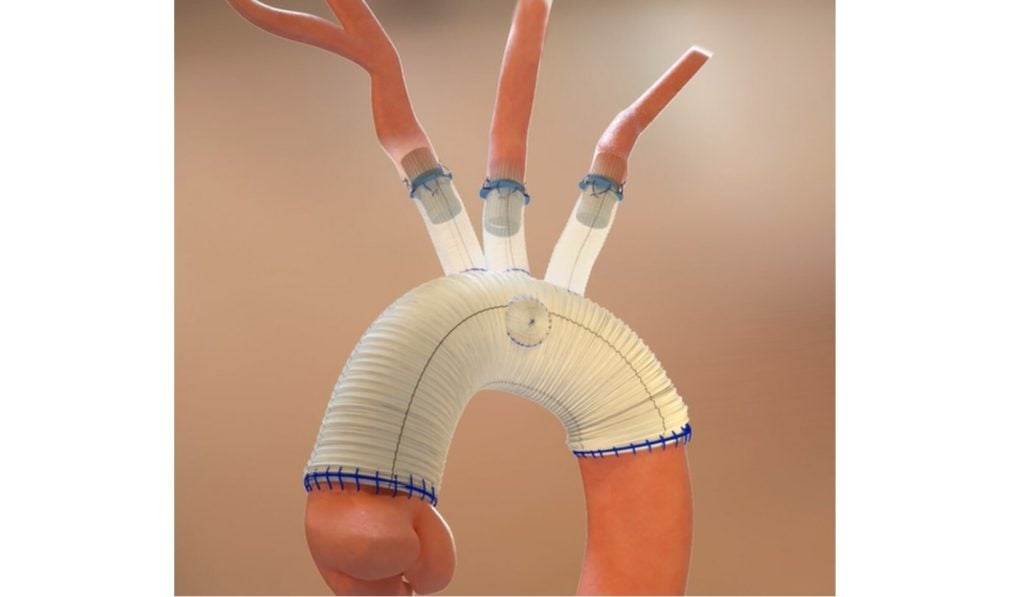
Aquedeon has designed the system to streamline and simplify open surgical procedures involving the thoracic aorta, helping cardiothoracic surgeons effectively treat the target vessels.
It has been designed to link native aortic arch branch vessels to the surgical graft without the necessity of circumferentially suturing each anastomosis.
This could potentially reduce the use of deep hypothermic circulatory arrest and shorten the overall procedure duration.
Aquedeon Medical chief operating officer Tom Palermo said: “The Duett Vascular Graft System has been developed in collaboration with leading cardiothoracic surgeons and the device is aimed at helping to address the complexities and intricacies of thoracic aortic surgeries.
“Reducing anastomosis time is one major step in addressing this long and complex procedure.
“We acknowledge the FDA's dedication during the IDE review process and we will continue to work closely with the agency throughout the study with a mission to put this innovative device into the hands of surgeons.”
Aquedeon is focused on the development of patented technologies for cardiothoracic surgical procedures.