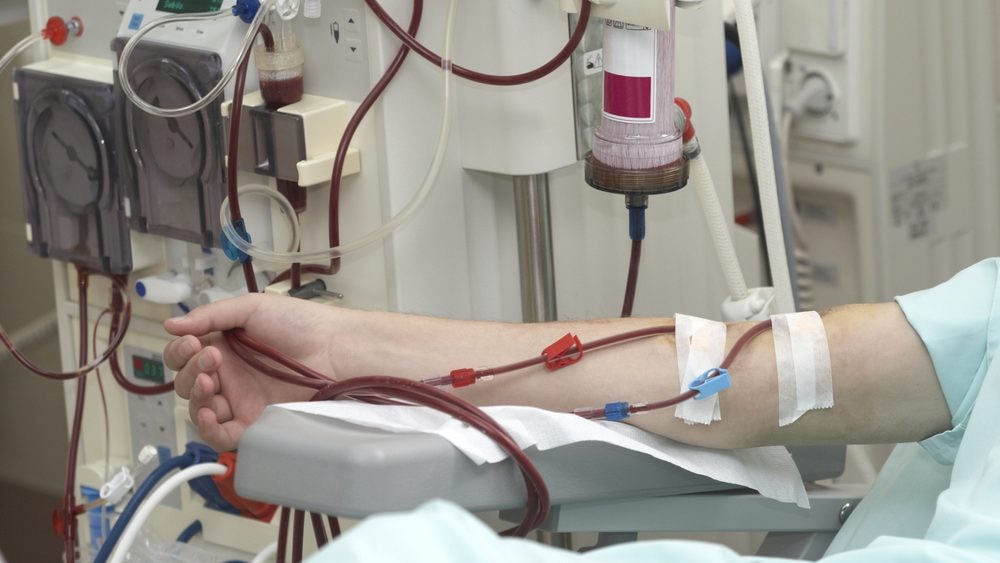
Baxter has announced positive data showing that patients using its expanded haemodialysis therapy, HDx with the Theranova dialyser, had a lower risk of dying after four years compared to those on traditional high-flux haemodialysis (HF HD).
The 48-month observational cohort study showed that 1,092 dialysis patients using HDx therapy had about a 25% lower risk of dying from any cause over four years, compared to HF HD users. The study was conducted at 11 Baxter renal care services centres in Colombia.
The expanded HDx therapy, used with Baxter’s Theranova dialyser, employs an advanced medium cut-off (MCO) membrane which can filter out a broader range of molecules, including large-middle molecules which conventional HF HD struggles to remove. These molecules are linked to inflammation and cardiovascular disease, so efficient removal can lead to better patient outcomes.
Baxter won de novo clearance from the US Food and Drug Administration (FDA) for the Theranova cartridge in 2020 and was launched outside of the US in 2016.
Peter Rutherford, vice president of kidney care at Baxter, said: “It is very exciting to see the results of this four-year study, indicating approximately 25% lower mortality for chronic kidney patients with the use of Baxter’s Theranova dialyzer. Dialysis treatments that more closely mimic the natural kidney have been associated with lower comorbidities, offering a protective effect for kidney patients.”
Haemodialysis is a procedure that uses a machine to filter waste and excess fluids from the blood when the kidneys can no longer do so. According to a market model on GlobalData’s Medical Intelligence Center, the US dialysis market will generate $901m in 2033.
There has been recent innovation in the dialysis space. In February 2024, Outset Medical entered a multi-year agreement with US Renal Care to provide patients and caregivers access to home dialysis with the Tablo haemodialysis system, to meet the increasing demand for home dialysis solutions. Tablo functions as a mobile dialysis clinic due to the integration of water purification and on-demand dialysate manufacturing.
In September 2023, AWAK Technologies secured more than $20m in Series B funding for the US-based trial of its wearable AWAK PD device.
The ultra-portable peritoneal dialysis system allows kidney disease patients to undergo dialysis at home and anywhere on the go. The trial is anticipated to kick off in 2025 in the US.