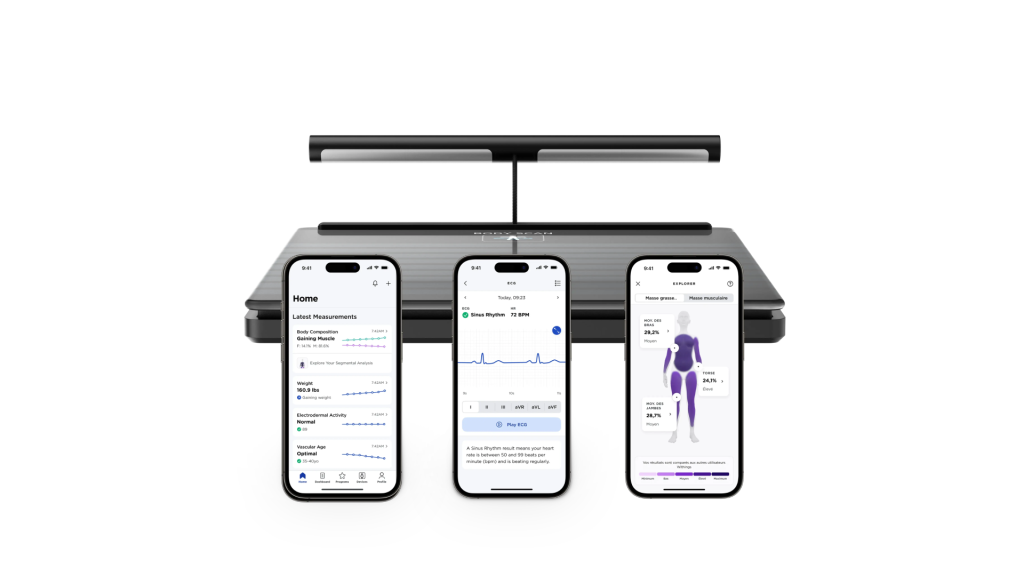
Withings, has announced it has obtained US Food and Drug Administration (FDA) clearance for its Body Scan Health Station in conjunction with the Scan Monitor 2.0.
The device provides daily data on biomarkers such as segmental body composition, heart rate, and vascular age. It uses a 6-lead electrocardiogram (ECG) to monitor nerveactivity and signs of atrial fibrillation (AFib) making the smart scale a ‘full-on home health station’.
Eric Carreel, President of Withings said: “With Body Scan, we will turn the morning weigh-in into a sophisticated home health check with access to holistic health data and personal health programs created by medical professionals. We will empower our users with the ability to take meaningful actions based on medical-grade data, adding a new dimension to ongoing lifestyle and chronic condition management through the ultimate in-home health experience.”
Being one of the most common cardiac arrhythmias, AFib is responsible for over 450,000 hospitalisations a year in the US alone.
The device, which uses electrode sensors located in the scale and retractable handle, gives the user a long-term picture of their heart patterns associated with AFib on the app and can be easily shared with healthcare professionals.
The vascular age function of the device uses a patented technology developed by Withings and allows the patient to assess and compare their cardiovascular health to the norm within their age bracket.
The sensors in the smart scale can measure Basal Metabolic Rate (BMR) detecting the number of calories the body burns at rest. It also uses multi-frequency Bioelectrical Impedance Analysis (BIA) to measure whole-body fat and water percentage, visceral fat, muscle, and bone mass and provides readings for individual body parts, including torso, arms, and legs.
At the start of 2023 the company announced the launch of U-Scan, the world’s first hands free connected home urine lab after four years of development. Promising to bring biomarker assessments to the home.
The Body Scan smart scale will become commercially available at the end of September 2023.