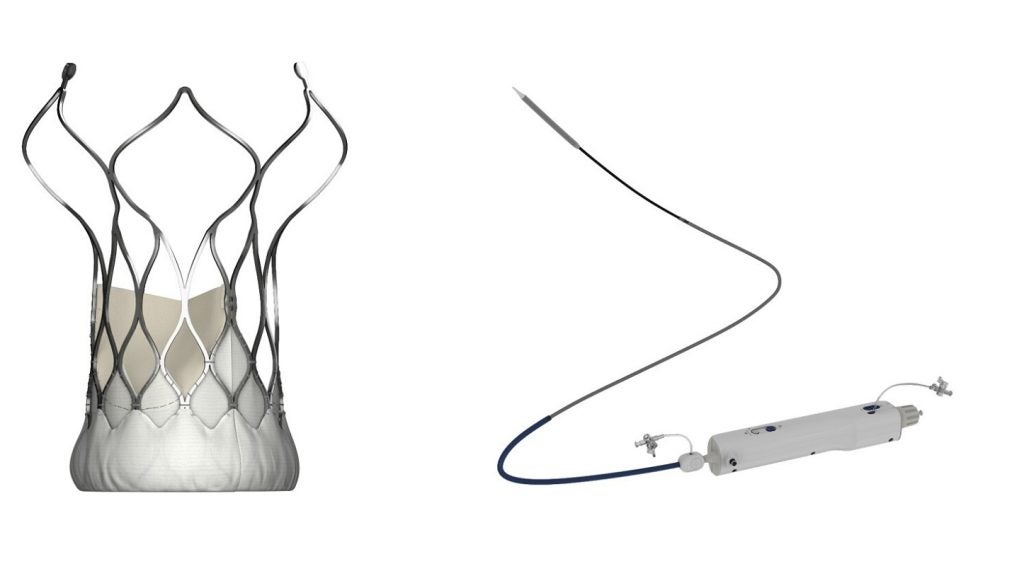
MicroPort CardioFlow Medtech (CardioFlow) has secured EU CE-MDR certification for its VitaFlow Liberty Transcatheter Aortic Valve and Retrievable Delivery System (VitaFlow Liberty).
This marks a major milestone for the second-generation transcatheter aortic valve implantation (TAVI) device for heart valve treatments.
CardioFlow chairman Guoming Chen said: “Securing the EU CE-MDR marking for VitaFlow Liberty is not just a passport for the product's entry into the European market, it also represents a significant milestone in CardioFlow's history and global roadmap.
“This achievement will assist in diversifying the company's sources of sales revenue and bolstering our overall competitiveness with a steadfast commitment to world-class product innovation.”
At the PCR London Valves 2023 conference, CardioFlow presented clinical data demonstrating the VitaFlow series valves' alignment with top-tier international standards.
In patients with severe aortic stenosis, VitaFlow’s long-term clinical performance showed encouraging results in cardiac mortality, all-cause mortality and permanent pacemaker implantation rates over a period of seven years.
The VitaFlow Liberty system is designed for flexibility and 360° range of motion, even in patients with complex anatomical challenges such as severe angled aortic arch deformities.
Its feature allows for the valve to be fully retrieved and repositioned up to three times during a procedure, enhancing the implantation process.
Prior to its market entry in the EU, the VitaFlow Liberty underwent pre-market clinical implantations in several European hospitals.
These included Galway University Hospital, Rigshospitalet, St Thomas' Hospital and Brighton & Sussex University Hospitals NHS Trust.
Additionally, CardioFlow has planned a European post-market clinical project, set to commence this year.
The company's global expansion roadmap also includes CE applications for other products such as the Alwide Plus Balloon Catheter and the Anchorman Left Atrial Appendage closure and access systems.