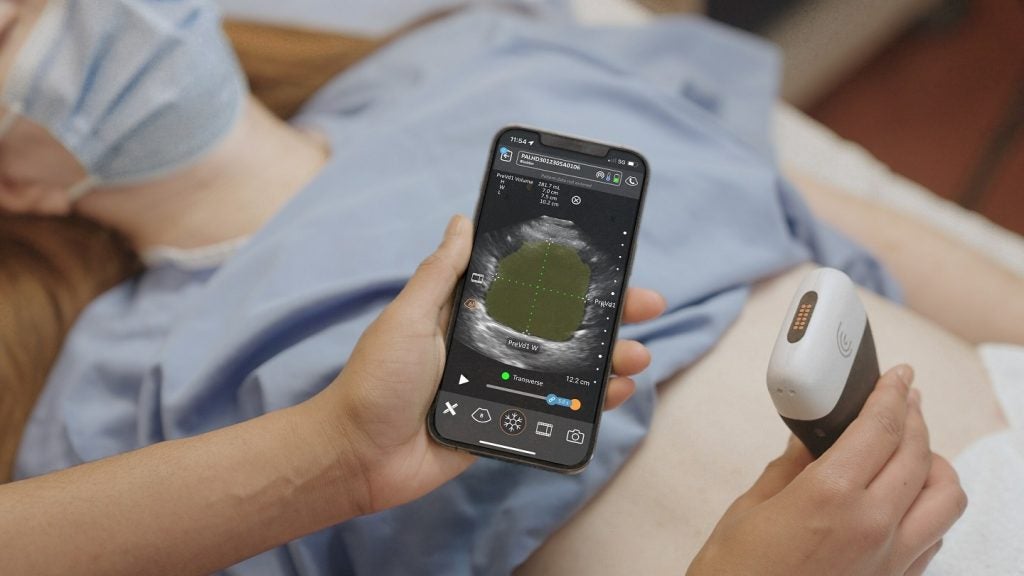
Clarius Mobile Health has received the US Food and Drug Administration (FDA) 510(k) clearance for its Clarius Bladder AI [artificial intelligence] non-invasive tool designed to automatically measure bladder volume.
The tool serves as a versatile and cost-effective alternative to traditional bladder scanners and provides real-time feedback to clinicians.
Clarius is offering the Clarius Bladder AI for acute care, urology, and nursing. It is launched in the US with the Clarius PAL HD3, Clarius PA HD3, and Clarius C3 HD3 models.
Clarius chief technology officer Kris Dickie said: “We made the decision to develop Clarius Bladder AI in response to the increasing demand from health systems for multi-function devices that can be used by physicians, nurses, and other ancillary staff for ultrasound-guided peripheral IV insertions and bladder scanning.
“Paired with our new Clarius PAL HD3, which offers the capability to image deep anatomy and superficial anatomy, it's a more cost-effective and versatile tool than current bladder scanners.”
Clarius Bladder AI, which is the latest addition to the Clarius AI portfolio, can be used for monitoring urinary retention and assessing bladder emptying in patients with conditions such as neurogenic bladder or urinary tract obstruction.
It is included in the Advanced Primary / Critical Care Package, which forms part of the Clarius membership.
Existing Clarius members with compatible scanners can access the new application immediately.
Last year, Clarius collaborated with Usono to launch a new wireless and wearable ultrasound imaging solution for researchers to study people’s anatomy while they move.
The solution integrates Clarius HD3 high-definition wireless ultrasound scanner with research software and the ProbeFix Dynamic device of Usono.
It allows for stable and hands-free ultrasound imaging during exercise and other activities where motion and continuous scanning play a significant role.