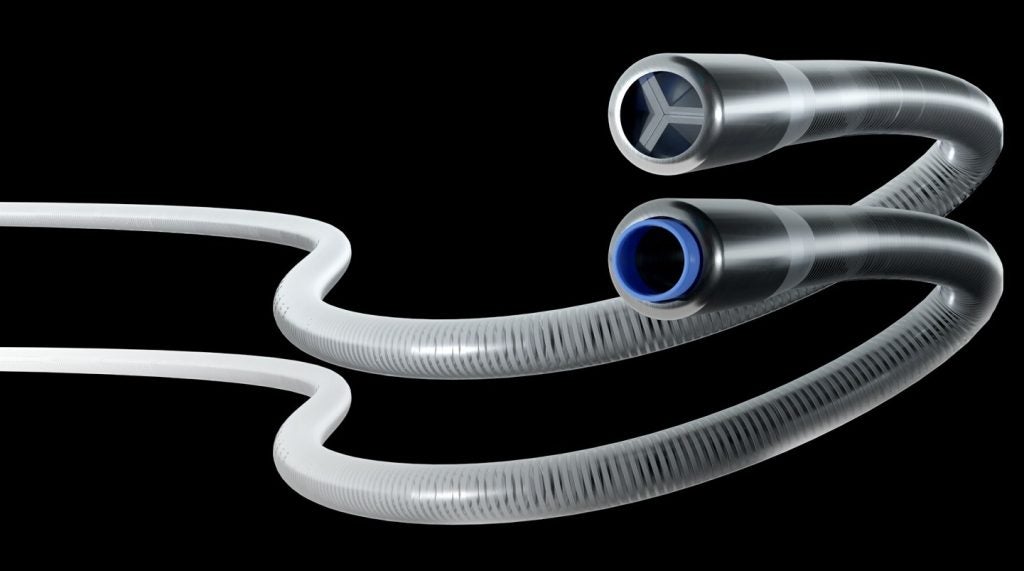
Concept Medical has announced the enrollment of the first patient in its MAGICAL-ISR clinical study in the US, evaluating the effectiveness of its MagicTouch Sirolimus drug-coated balloon (DCB).
Intended for treating in-stent restenosis (ISR) in coronary artery disease, the MagicTouch Sirolimus DCB earlier received a breakthrough device designation and investigational device exemption (IDE) approval from the US Food and Drug Administration (FDA).
As part of the MAGICAL-ISR study, the patient enrollment commenced at the AtlantiCare Institute in Atlantic City, New Jersey.
The study's primary objective is to determine the safety and efficacy of the Sirolimus DCB in managing ISR, with a focus on the proportion of patients who avoid target lesion failure (TLF) within one year after the procedure.
Physicians Dr Martin Leon, Dr Azeem Latib, and Dr Ajay J Kirtane are guiding the study.
Cardiovascular Research Foundation (CRF), New York founder and study chair Dr Martin Leon said: “We are thrilled to announce the first enrollment of the MAGICAL-ISR study featuring the MagicTouch Sirolimus drug-eluting balloon technology.
“This momentous clinical trial will herald an era of safe and efficacious DEB therapy under a variety of clinical and anatomic circumstances to complement and improve the management of complex obstructive coronary disease for our patients in the United States.”
MagicTouch is said to be a significant innovation in coronary intervention. It offers a controlled and sustained release of Sirolimus.
It also provides the benefits of a drug-eluting stent without the need to implant a permanent scaffold.
Concept Medical founder and managing director Dr Manish Doshi said: “The MagicTouch technology has been embraced globally, and its entry into the US market through this study, represents a critical step towards addressing the unmet needs in ISR treatment.”