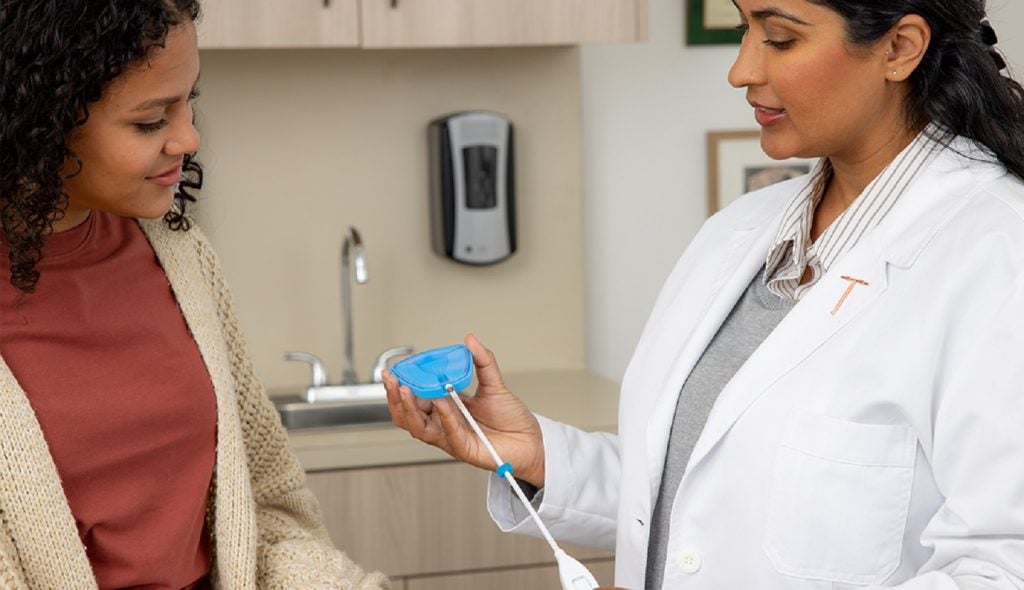
CooperSurgical has introduced a new single-hand inserter for the Paragard copper intrauterine device (IUD).
The device recently received approval from the US Food and Drug Administration (FDA).
The company’s latest innovation is set to streamline the placement process for healthcare providers, enhancing the accessibility of Paragard as a contraceptive option.
It is designed to offer healthcare professionals a simpler way to place the Paragard IUD.
With more than 30 years of clinical use, Paragard is known for being a non-hormonal contraceptive, providing more than 99% efficacy for up to ten years and is immediately reversible.
CooperSurgical president Holly Sheffield said: “We are constantly striving to meet the evolving needs of healthcare providers, and the patients they serve.
“With nearly eight million women choosing Paragard, the new inserter simplifies the placement process and marks a significant milestone for the product. It reaffirms our mission to deliver innovative and safe solutions that positively impact the lives of patients and providers everywhere.”
A market survey has indicated high satisfaction rates among Paragard users, with 91% of women aged between 25 and 50 years expressing contentment with the product after using it for at least one year.
The new inserter, equipped with a built-in loading tip, aims to maintain the reliability of Paragard while simplifying the insertion procedure.
The commercial availability of Paragard with the inserter marks a significant development in the contraceptive market.
As a hormone-free IUD, it offers a long-term pregnancy prevention solution, effective for up to ten years, utilising the contraceptive properties of copper.
CooperSurgical, a subsidiary of CooperCompanies, has a portfolio of more than 600 medical devices. These products cater to a range of needs within women’s healthcare, including various testing and treatment options.