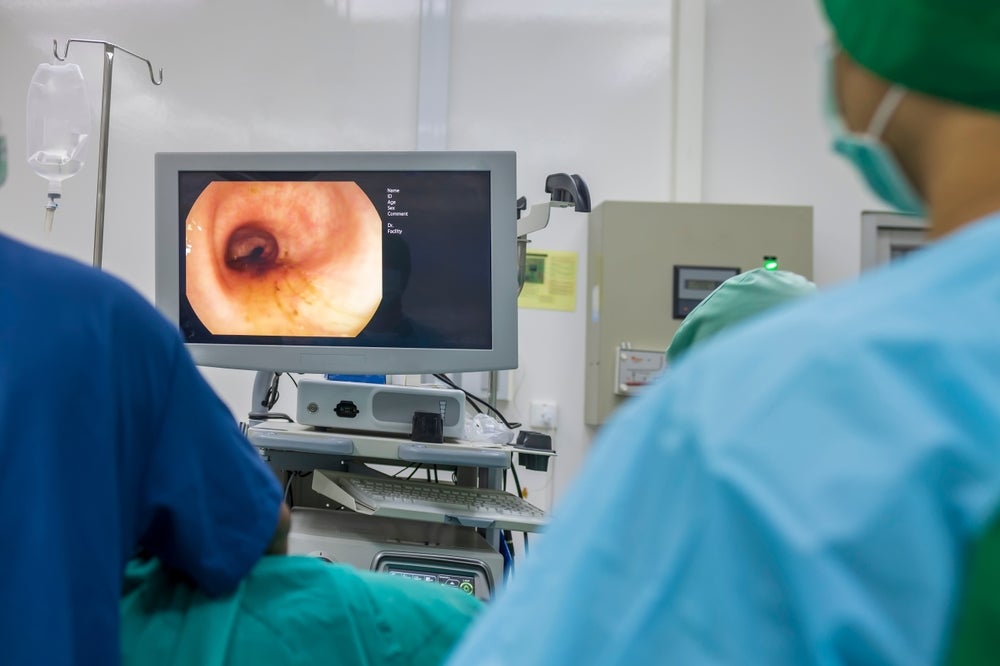
UK-based company Creo Medical is planning the launch of its Speedboat UltraSlim device, 18 months ahead of schedule.
Speedboat UltraSlim will be the second product in the Speedboat line and follows its predecessor Speedboat Inject.
The device is designed for flexible endoscopy procedures, targeting therapeutic treatment of Gastrointestinal (GI) tract lesions, including Bowel and Upper GI cancers, and pre-cancerous lesions and swallowing disorders. New features include a reduced size that allows compatibility with all endoscopes with a 2.8mm working channel or larger.
It will be powered by Creo Medical’s CROMA advanced energy platform and will deliver bi-polar radiofrequency energy for precision cutting and high frequency microwave energy for controlled coagulation of tissue in the GI tract.
The original plan for the launch of Speedboat UltraSlim would have seen the device released in 2025 but following a submission to the company’s notified body which advised on the regulatory pathway which facilitates this early launch.
Craig Gulliford, CEO of Creo Medical, said: “The regulatory landscape has been particularly challenging for all medtech companies in the last few years here in Europe, with changing transition deadlines and elongated and uncertain clearance pathways. This has resulted in most companies seeking clearance now in the US ahead of the EU. We had adopted the same approach, planning to launch the device in the US ahead of the EU, having filed for our FDA clearance earlier this year.”
Elsewhere there has been an increased popularity of Capsule Endoscopes. In the North America the number of Capsule Endoscopy Procedures performed was 153,641 in 2015 and GlobalData predicts it to reach 667,406 in 2030. While in the UK the number of Capsule Endoscopy Devices performed grew from 34,968 in 2015 and is forecast to reach 966,665 in 2030.