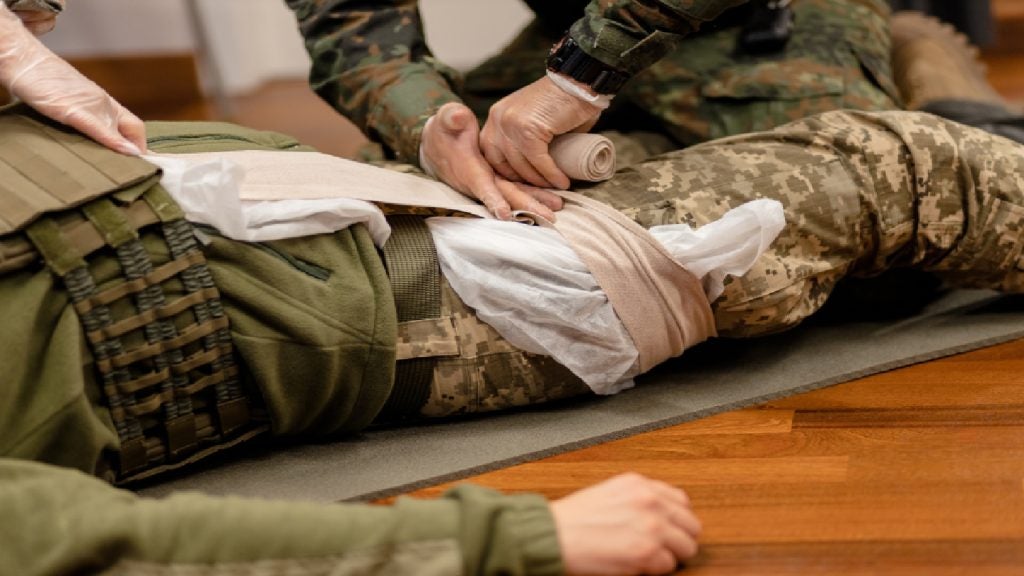
The US Food and Drug Administration (FDA) has granted market clearance to a medical gel designed to stem bleeding in emergency situations.
The FDA has granted New York-based company, Cresilon, a second 510(k) clearance for the plant-based gel, known as Traumagel, for the temporary external use for controlling moderate to severe bleeding, with the aim of supplying it to the US armed forces and emergency services. The company received a first clearance in June 2023, for local management of bleeding wounds.
According to Cresilon, the gel is designed to directly stem the bleeding of wounds caused by severe trauma, for example, a gunshot wound, with the gel hardening on contact to form a seal around the point of injury.
Research published by the US National Institutes of Health (NIH) found trauma to be the leading cause of death worldwide among people younger than 44. Up to 60% of those who die from blood loss stemming from a traumatic injury pass within the first three hours following the incident. Cresilon hopes that its trauma gel will be used in place of traditional gauze and bandages.
Joe Landolina, CEO, and co-founder of Cresilon said: “The ability to rapidly stop bleeding at the point of care and halt a life-threatening haemorrhage can be the difference between life and death for people with traumatic wounds.
“The FDA clearance for Traumagel is a monumental milestone for Cresilon and brings us another step forward in our mission to save lives and transform the standard of care in wound treatment. Our proprietary hemostatic gel technology is a game-changer and unlike any other hemostatic agent currently being used."
A market growth model by GlobalData estimates that the wound care management market will be worth nearly $39bn by 2030. Previously the gel had been given the greenlight to be used in the veterinary industry before receiving clearance from the FDA for its first human use in June 2023.
In July 2024, the company completed the first phase of a preclinical study alongside the US Department of Defense (DOD) examining the impact of the gel in the treatment of head and brain injury.