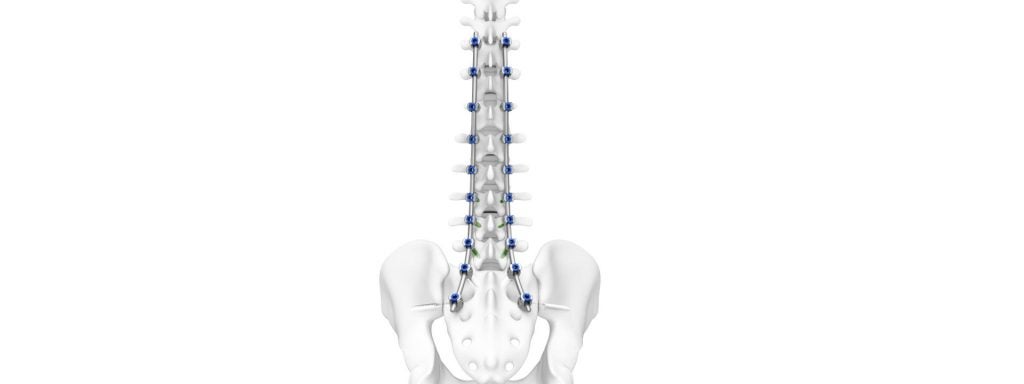
DePuy Synthes a Johnson & Johnson orthopaedics company has been awarded US Food and Drug Administration (FDA) 510(k) clearance for its thoracolumbar implant system, TriATLIS Spine System, and its Navigation Enabled Instruments.
The TriATLIS Spine System is a thoracolumbar pedicle screw system with a portfolio of instruments compatible with DePuy Synthes’ enabling technologies. It is designed to assist surgeons treating spinal issues such as inclusive of degenerative, tumour, trauma, and deformity pathologies.
DePuy Synthes claims spinal deformity diseases affects 32% of young adults and 68% of adults over the age of 65. And with an ageing populations surgeons are seeing a wider scope of deformities that require spinal fusion instruments.
Daniel Sciubba Professor of Neurosurgery at Northwell Health/Hofstra praised the portfolio of implants from TriATLIS he said: “When it comes to treating complex spinal pathologies, each patient is different, and each case requires its own unique treatment decisions.”
Following the clearance, the TriATLIS Spine System will be available for the US market in 2024.
According to GlobalData, the surgical sutures market is growing at 3.4% annually to be worth $4.5 billion by 2033, up from a value of $3 billion in 2022.
In August DePuy Synthes secured FDA 510(k) Clearance for its INHANCE Shoulder System and 510(k) Clearance for MAXFRAME Multi-Axial Correction System.