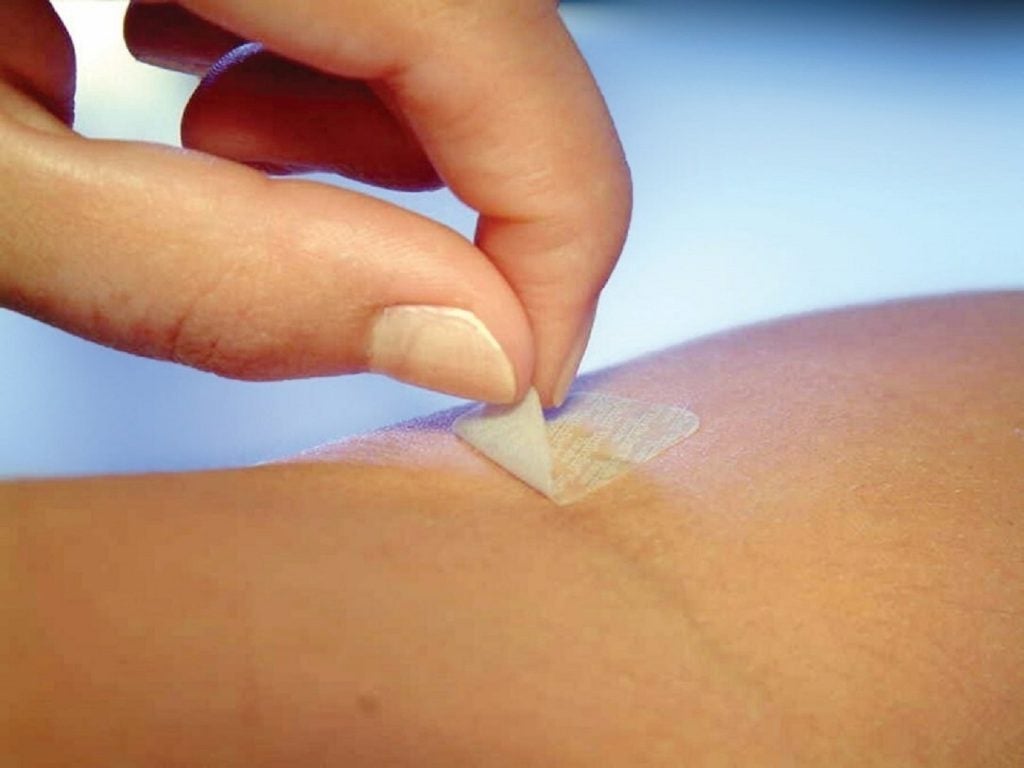
Technology-based materials and solutions provider DuPont has launched a new higher-adhesion, low-cyclic silicone soft skin adhesive (SSA) called DuPont Liveo MG 7-9960.
The new adhesive has been developed to be used for advanced wound care dressings and adhering medical devices to the skin for extended wear time and their removal in a gentle manner.
DuPont Liveo is a provider of silicone technology for medical devices. These technologies have been widely used in the market for advanced wound care and scar therapies.
The new adhesive is claimed to have the highest peel adhesion in the Liveo SSA family.
It is said to have high repositionability, enhanced wear performance, design flexibility, better conformability and atraumatic removal.
Claimed to be a non-sensitising, non-irritating and non-cytotoxic adhesive, DuPont Liveo MG 7-9960 helps to protect the highly sensitive and fragile skin of populations including children, the elderly and patients with skin conditions or open wounds.
Manufacturers can adjust the coat weight of DuPont Liveo MG 7-9960 to meet the specific application requirements of device designers, allowing for fine-tuned performance.
DuPontLiveoHealthcare Solutions global strategic marketing leader Jennifer Gemo said: “As difficult-to-heal wounds are increasingly prevailing and wound-healing rates need to improve, we are looking at expanding our portfolio of medical-grade adhesive solutions and partnering with medical device OEMs and converters to support them in developing their new dressing or wearables designs.”
In May this year, DuPont signed a definitive agreement to acquire speciality medical device maker Spectrum Plastics Group from AEA Investors for $1.75bn.