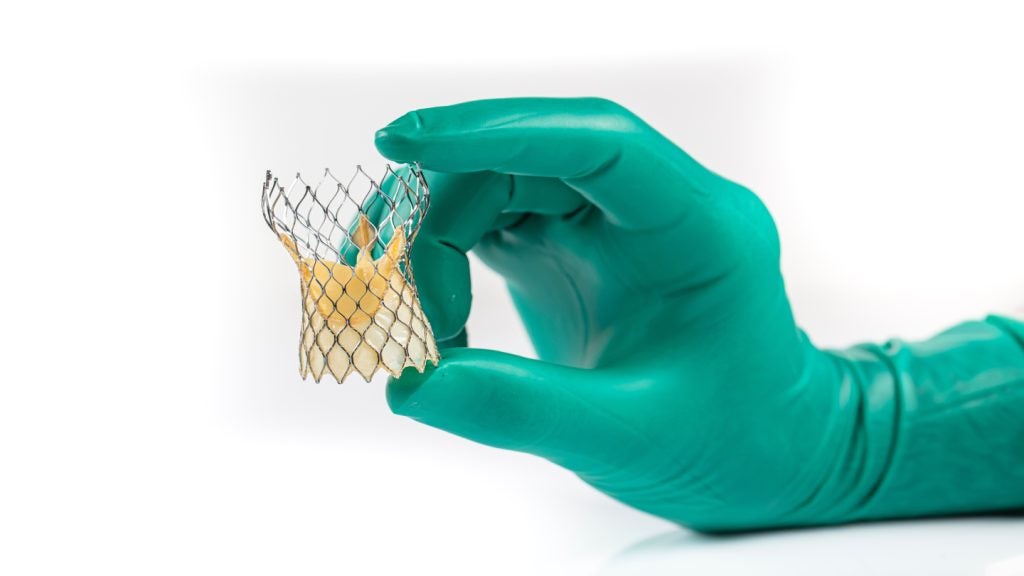
Edwards Life Sciences, has announced positive results from its PARTNER trial examining its transcatheter aortic valve replacement (TAVR) system designed for patients with a smaller than usual annulus, the interior lining of a heart valve.
A prothesis-patient mismatch (PPM) is when the TAVR system is physically mismatched with the shape or size of the interior of a patient’s artery, liable to result in poorer long-term outcomes or death, disabling stroke or heart failure-related hospitalization.
Edwards says that the five-year follow-up of more than 1,300 low and intermediate-risk SAPIEN 3 valve patients saw very positive outcomes low rates of reintervention and high survival rates within five years from the start of the study. Edwards additionally says that the PARTNER 3 trial demonstrated the highest reported survival rate in low-risk patients seen in any pivotal trial in the company’s history.
Rebecca Hahn, investigator for the trial, said: “This important dataset highlights the risk of relying on a singular hemodynamic parameter such as mean gradient as a surrogate for valve dysfunction.
“When selecting the best treatment option, we must evaluate measures that matter to patients such as death, disabling stroke, quality of life and reintervention. This five-year follow-up of low and intermediate-risk patients demonstrated no prosthesis-patient mismatch. This is one of the key outcomes for the Edwards SAPIEN 3 platform. These are important findings for clinicians in determining the best treatment for patients.”
The data comes as Edwards Life Sciences released data from additional studies that found its SAPIEN 3 Ultra and SAPIEN transcatheter aortic valves saw lower rates of paravalvular leak (PVL), with 88.3% of patients experiencing no PVL at all.
Larry Wood, Edwards’ corporate vice president, added: “The PARTNER series of robust pivotal trials, all of which included FDA oversight, rigorously followed more than 12,000 patients treated with Edwards SAPIEN valves rendering them excellent data from which to examine the totality of factors that contribute to valve durability and performance.
“These data are reassuring for patients and clinicians – particularly women who are more likely to receive a smaller valve – that the SAPIEN platform offers excellent survival and very low reintervention rates at five years.”
Elsewhere in the growing field of TAVR treatment, the US Food and Drug Administration (FDA) has approved Medtronic’s Evolut FX+ TAVR system, designed for the treatment of symptomatic severe aortic stenosis.