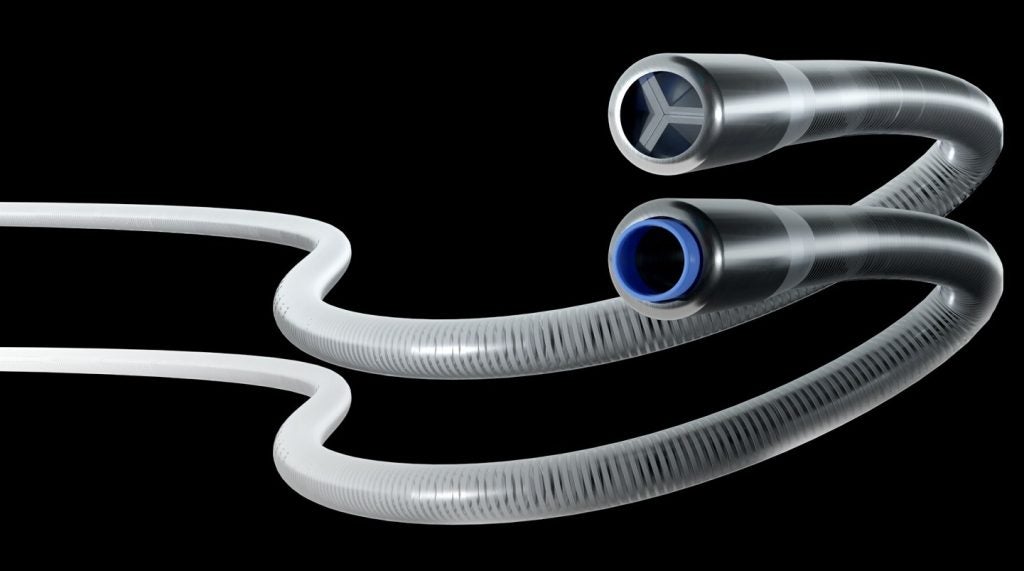
The US Food and Drug Administration (FDA) has issued 510(k) clearance to Expanse ICE’s aspiration thrombectomy system ICE Aspiration System.
The FDA clearance marks a significant milestone in the medical device sector, particularly in the field of thrombectomy treatments, stated the company.
The ICE Aspiration System is designed to help address the challenges related to peripheral thrombectomies. Blood clots are said to be the third leading type of vascular disease.
It features a dual-lumen design to create the patented Surge Aspiration technology, a novel cyclic aspiration type at the distal end of the catheter.
Expanse ICE vascular device field serial entrepreneur Dr Eitan Konstantino said: “We've engineered the ICE catheter system to harness the aspiration power typical of a large bore catheter, but within the slender profile of a much smaller device.”
Vascular Center Clinic in Arnsberg, Germany, angiology chief medical officer Dr Michael Lichtenberg said: “It is clear this device was built with physicians in mind. It aims to address some of the biggest issues we see regularly.
“The device was designed and built with a strong understanding of foundational physics that leaves me optimistic for its success.”
According to the US Centers for Disease Control, approximately one million patients face peripheral blood clots that must be treated annually, and up to 33% of them suffer complications in the long term.
Cleveland Clinic peripheral vascular interventions director Dr Aravinda Nanjundappa said: “I am looking forward to being among the first users of the Expanse ICE system. Its ability to offer powerful clot removal in a compact size is an intriguing proposition that could significantly enhance our ability to meet patient needs more effectively and improve treatment outcomes in peripheral vascular disease.”