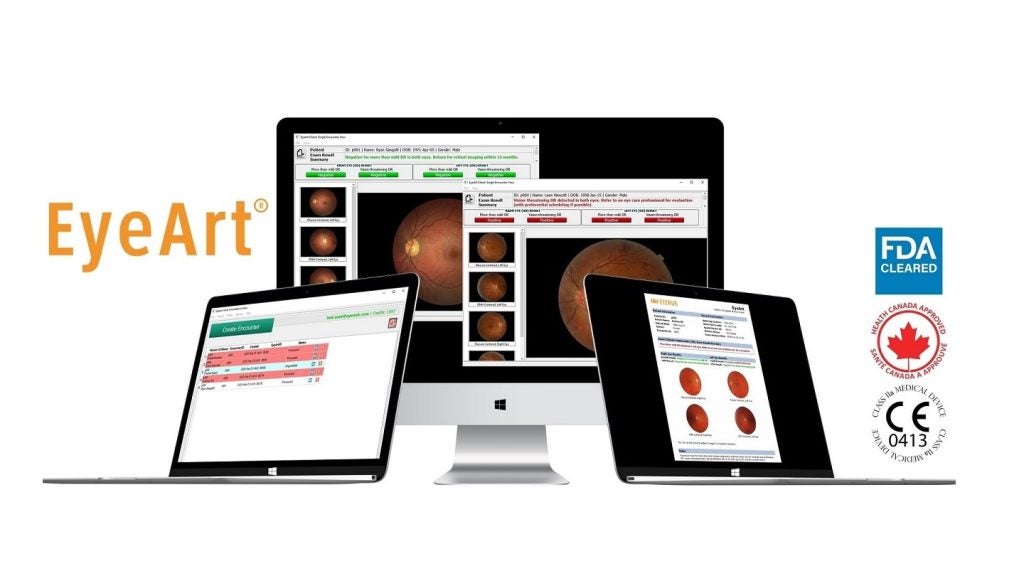
Artificial intelligence (AI) digital health corporation Eyenuk has secured approval from the US Food and Drug Administration (FDA) for its EyeArt v2.2.0 system.
The approval expands the label of the EyeArt AI system, the company’s flagship product.
It enables the use of Topcon NW400 retinal camera with the EyeArt AI system to automatically identify diabetic retinopathy (DR).
This system is already approved for use with Canon CR-2 AF and Canon CR-2 Plus AF cameras.
The EyeArt v2.2.0 system is claimed to be the first and only AI system approved by the US regulator for use with several retinal cameras by different manufacturers.
The system’s latest approval is based on clinical data from a multi-centre study, which showed superior performance for the Topcon NW400 cameras.
Furthermore, the approval allows the company to add its Real-Time Image Quality Feedback solution and an upgraded image quality assessment module to the EyeArt system.
Eyenuk founder and CEO Kaushal Solanki said: “The EyeArt system can now be used with multiple camera models in the US, which significantly expands access for patients who can be screened in their primary care doctor’s office for preventable blindness due to diabetes.”
In August 2020, the company launched the EyeArt system, which was approved by the FDA to identify more than mild and vision-threatening DR.
This system was approved under MDR Class IIb in the European Union to identify DR, glaucomatous optic nerve damage and age-related macular degeneration, in a single test.