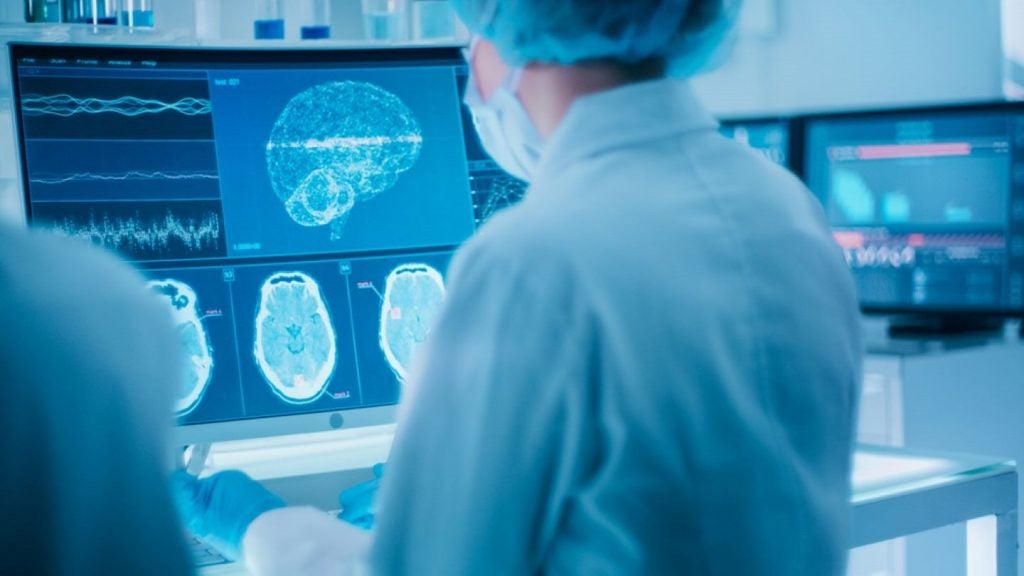
Ezra has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its new artificial intelligence (AI) technology, Ezra Flash, for use in brain imaging.
Ezra Flash is designed to improve the quality of magnetic resonance imaging (MRI).
The new AI technology will allow the company to launch the 30-minute full-body MRI scan, which is claimed to be the first of its kind across the world.
A rapid MRI protocol that was developed by the company generates noisy images. Ezra Flash can address this issue by improving the quality of these images.
Ezra founder and CEO Emi Gal said: “Our mission at Ezra is to detect cancer early for everyone in the world and I’m really excited about this new AI enabling us to make our scan more affordable.
“By boosting quality while reducing scan time, we’re decreasing our cost for a full body MRI by 30% and we’re passing these cost savings to our customers.”
The Ezra Flash AI underwent training by leveraging the company's longitudinal MRI dataset, encompassing a vast collection of MRI images obtained from both healthy individuals and patients.
Ezra trained the AI to identify the crucial components of an MRI scan that are required for producing a complete and precise image.
The latest FDA clearance marks a significant milestone for Ezra as it becomes the first global company to deploy AI across imaging, analysis and reporting aspects of the cancer screening process.
The company’s Ezra Prostate AI enables radiologists to analyse MR images of the prostate.
It also offers Ezra Reporter AI that generates an easy-to-understand radiology report, which can be used by medical professionals to share screening results with individuals.