The US Food and Drug Administration (FDA) has granted approval for Boston Scientific’s cryoablation system, dubbed POLARx.
The system is intended for treating paroxysmal atrial fibrillation (AF) patients.
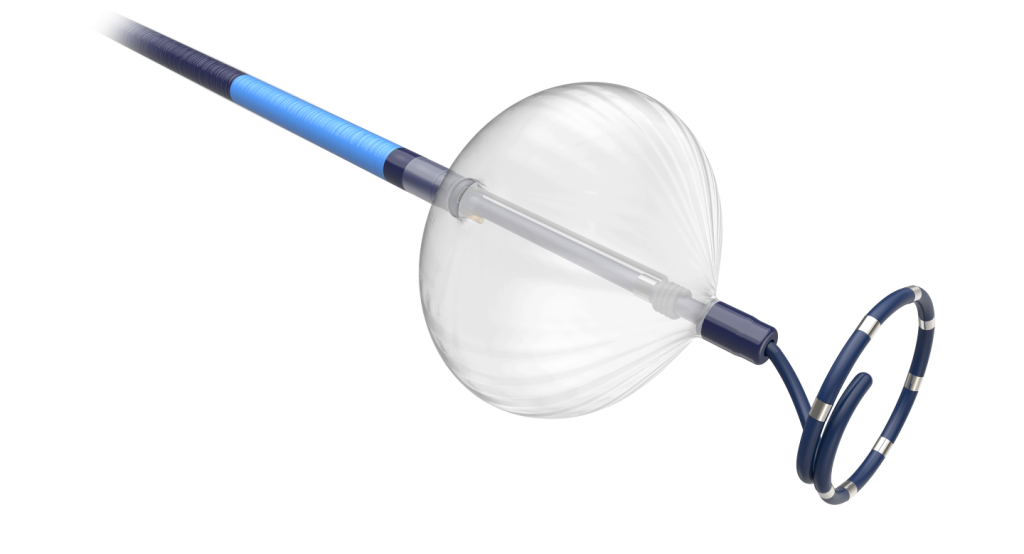
It features the POLARx FIT cryoablation balloon catheter built with the ability to enable two balloon sizes, 28mm and 31mm, in a single catheter.
A minimally invasive procedure, cryoablation facilitates AF treatment by delivering cryotherapy to the pulmonary vein through a balloon catheter.
This approach freezes the affected tissue to create scarring, thereby hindering irregular electrical signals.
The new POLARx FIT catheter can be adjusted to fit the anatomy of patients during the procedure and helps address various limitations.
According to findings from the FROZEN-AF IDE clinical trial, POLARx was found to be safe and effective in treating paroxysmal AF patients.
At 12 months, the primary event-free rate was reported to be 96%, without any cases of persistent phrenic nerve palsy or oesophagal fistulas and moderate or severe pulmonary vein stenosis.
Boston Scientific electrophysiology president Nick Spadea-Anello said: “The US approval of the POLARx cryoablation system, which has been used in more than 25,000 patients worldwide to date, marks an exciting advancement for the treatment of AF and a new era of cryoablation capabilities.
“By prioritising procedural flexibility and individualised care, this offering transforms a key therapy in the electrophysiology space, addresses the unmet needs of physicians and affirms our commitment to making meaningful innovations to established technologies.”
The company recently posted GAAP net income attributable to its common stockholders of $261m in Q2 2023.