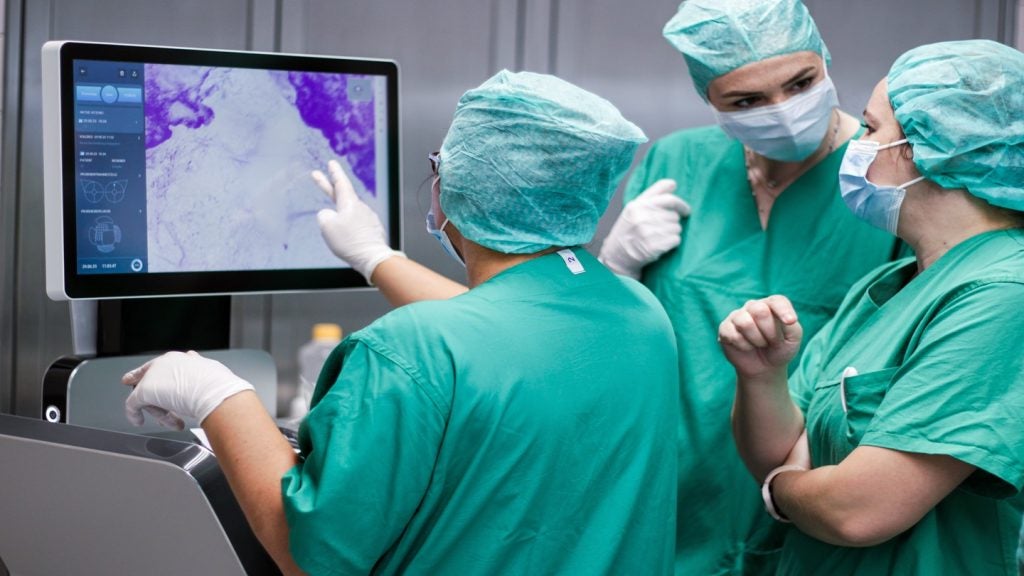
SamanTree Medical has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Histolog Scanner, a device that enables high-resolution imaging of fresh tissue surfaces in real-time.
This Swiss-made device provides detailed insights into the microstructure of tissues, aiding surgeons and pathologists during medical procedures.
The Histolog Scanner's capability for ultra-fast confocal microscopy allows for the immediate imaging of resected tissues, which is expected to enhance the efficiency of physicians.
By providing this information instantaneously, the device aims to assist in the decision-making process during surgical procedures.
With the FDA clearance secured, SamanTree Medical is now set to accelerate its market entry in the US.
SamanTree Medical executive chairman Dr Charles Carignan said: “We are thrilled to introduce the Histolog Scanner to the US market.
“This FDA clearance is a significant milestone for the company. The ability of the Histolog Scanner to produce real-time imaging of the internal microstructure of tissues provides surgeons and pathologists with immediate, actionable information.”
The company has already established a presence in Europe with a CE mark obtained for the Histolog Scanner in 2018.
In July 2024, SamanTree Medical secured $14m in funding to further the development and commercialisation of the Histolog Scanner in both Europe and the US.
The Series B funding round was spearheaded by Relyens Innovation Santé, advised by Turenne Capital, and saw contributions from new investors such as Mutuelles Impact, Wille Finance, Noshaq, and Wallonie Entreprendre Life Sciences. Existing investors Panakès Partners, BOM, and b2venture also participated.