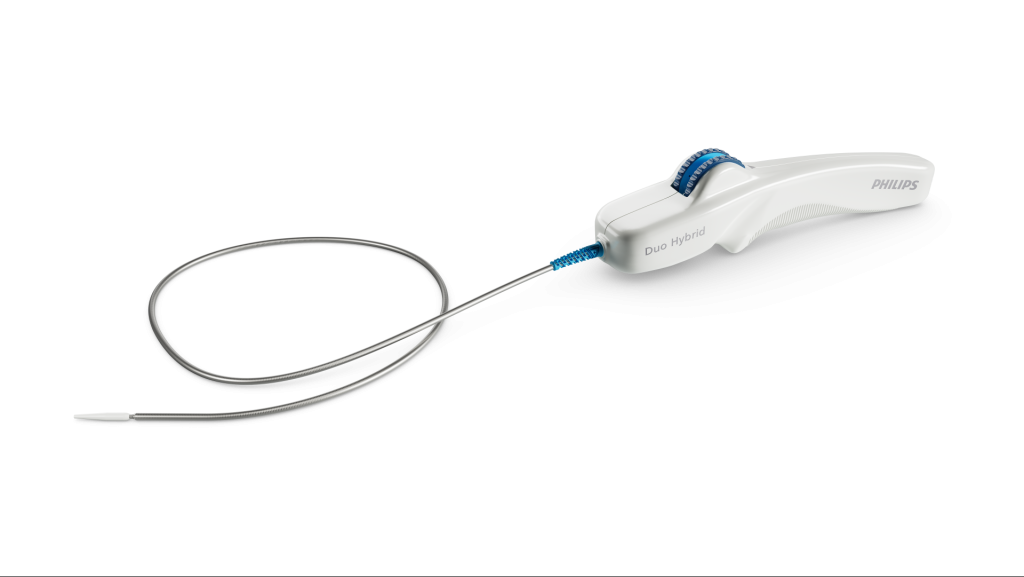
Philips’ Duo Venous stent system has been implanted into the first patient with symptomatic venous outflow obstruction in patients with chronic venous insufficiency (CVI), outside a clinical trial.
The Duo Venous Stent system comprises two stents, Duo Hybrid and Duo Extend. These stents are designed to work together, combining different mechanical properties into a single stent. The stents work together and minimise the risk of fracture and corrosion while serving caudal veins with smaller diameters.
The device won premarket approval from the US Food and Drug Administration (FDA) last year, following positive data from the VIVID study (NCT04580160). The 162-subject study met all of its primary safety and efficacy endpoints, with the safety endpoint of 98.7% coming ahead of the 89% target. Erin Murphy, investigator in the VIVID study, carried out the first procedure outside of the clinical trial.
Venous stents are small, expandable tubes inserted into veins to keep them open, ensuring proper blood flow and preventing blockages. They are often used to treat deep vein thrombosis (DVT) and CVI. According to a market model on GlobalData’s Medical Intelligence Center, the venous stent market will generate $253m in the US by 2030.
Philips vice president and peripheral vascular business leader Heather Hudnut Page said: “In this context, we look forward to bringing the combined offering of intravascular ultrasound and Duo to the interdisciplinary teams – from vascular surgeons to interventional radiologists and interventional cardiologists – who share our overarching goal of enhancing patient care.”
Last month, US-based device company AngioDynamics announced new data that found its AlphaVac F1885 System is safe in patients with acute intermediate-risk pulmonary embolism (PE) and provides significant improvement in right ventricular function and reduction in clot burden.
In January 2024, Endovascular Engineering secured an investigational device exemption approval from the FDA for a clinical trial of its Hēlo Thrombectomy System in treating pulmonary embolism. The system simultaneously aspirates and mechanically disrupts the blood clot.