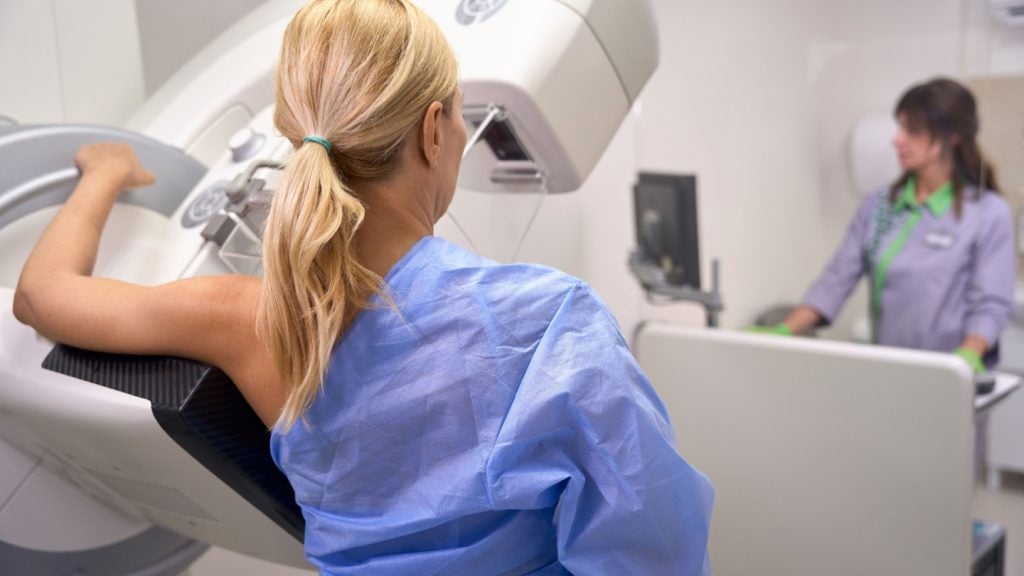
GE HealthCare has announced the new Pristina Via mammography system to improve technologist workflow and patient-centred breast care.
Pristina Via is set to offer advanced tools designed to balance diagnostic accuracy with rapid-paced workflows, allowing technologists to provide quality and personalised care at the time of screening.
The system features include a zero-click acquisition functionality allowing for rapid and seamless procedures. It also claims to provide no wait time between exposures, enabling technologists to work at their preferred pace.
The system's digital breast tomosynthesis (DBT) image-to-image cycle time is reportedly up to twice as fast when compared with other available mammography systems.
Additionally, Pristina Via offers vendor-neutral prior image comparison, which decreases the analysing time spent on earlier examinations. Notably, it claims the lowest radiation dose usage for every breast thickness compared to other systems.
The Pristina Via system has a scalable platform with full backward compatibility allowing imaging centres to access the latest capabilities.
GE HealthCare Women’s Health and X-ray CEO and president Jyoti Gupta said: “At GE HealthCare, we are dedicated to addressing our customers’ needs by delivering solutions that enhance the experience for both patients and healthcare professionals.
“Pristina Via represents a significant evolution in our patient-focused Senographe Pristina platform, which was designed by women for women.”
GE HealthCare global mammography general manager and vice-president Pooja Pathak said: “During our intensive collaboration with our end users in the design phase, we heard time and again that the technologist’s workday can feel like a race against the clock.
“Pristina Via is our answer to this challenge. The system’s advanced features were engineered to automate processes and allow our users to give patients the attentive, quality care they deserve. I am thrilled that SimonMed our longtime customer, will be implementing this new technology as part of their mammography services.”
During this month, the Food and Drug Administration (FDA) granted 510(k) clearance for the company’s SIGNA MAGNUS MRI scanner.