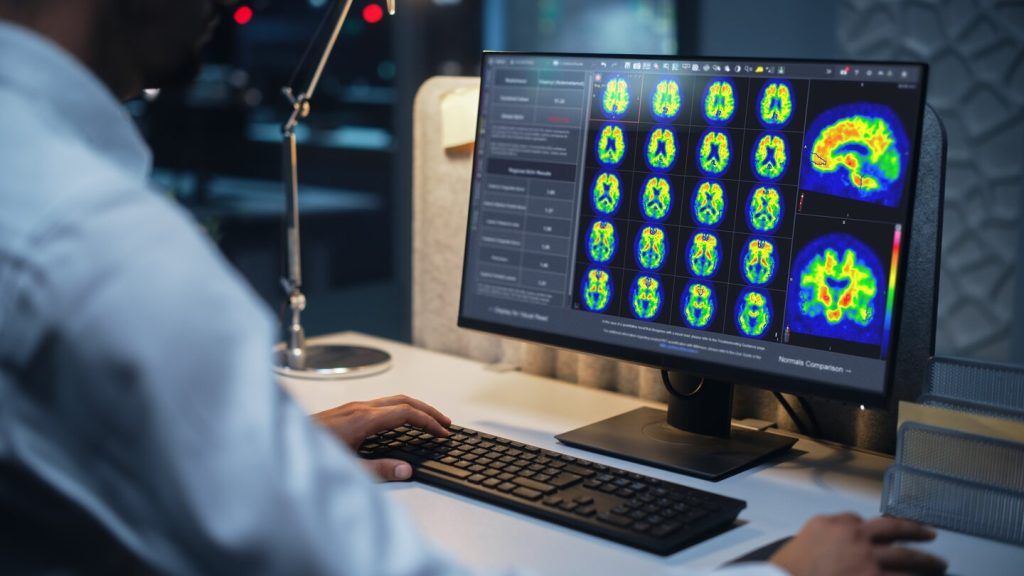
GE HealthCare’s (GEHC) MIM Software has secured 510(k) clearance from the US Food and Drug Administration (FDA) to offer its MIMneuro software with Centiloid scaling in patients being evaluated for Alzheimer’s disease.
MIM Software’s Centiloid scaling tool is approved for positron emission tomography (PET)-based amyloid imaging analysis and quantification. The approach aims to increase clinician confidence in determining amyloid plaque density - a key signifier of Alzheimer’s disease pathology - in a patient’s brain.
Centiloid scaling provides a standardised metric used to compare amyloid results, with zero representing the average value from high-certainty amyloid-negative patients and 100 representing the average value typically seen in patients who have Alzheimer’s disease.
The company claims that MIMneuro supports the Centiloid scaling tool by providing automated workflows that guide users through generating and understanding the Centiloid scaling result. Taking the PET amyloid images, the software provides clinicians with instructions to generate quantitative results, presenting the data in a standardised report for clinicians to review alongside the images.
GEHC MIM Software CEO Andrew Nelson commented: “Centiloid scaling with MIMneuro offers a standardised, quantitative metric to assist healthcare providers in confidently estimating amyloid plaque density, one key aspect of Alzheimer’s disease. By increasing clinician confidence, we hope to ultimately expand patient access to cutting-edge, personalised care.”
GEHC completed its acquisition of MIM Software, which provides a suite of imaging analytics and digital workflow solutions, in April 2024. As per the company, the acquisition aligns with its precision care strategy, with the overall aim of delivering a range of digital solutions across care pathways for more precise, connected, and efficient care across disease states.
GlobalData forecasts that the Alzheimer’s disease market will be worth $15.9bn globally by 2030, with more than 70 in vitro diagnostics for Alzheimer’s currently in development.
This year, the Alzheimer’s space has seen a range of activity in the development and launch of Tau biomarker tests. Along with amyloid beta, Tau is a key protein implicated in the disease.
In April, Roche secured FDA breakthrough designation for its Tau biomarker test, and Genova and Neurocode partnered for the development of a Tau blood test for early Alzheimer’s detection last month.