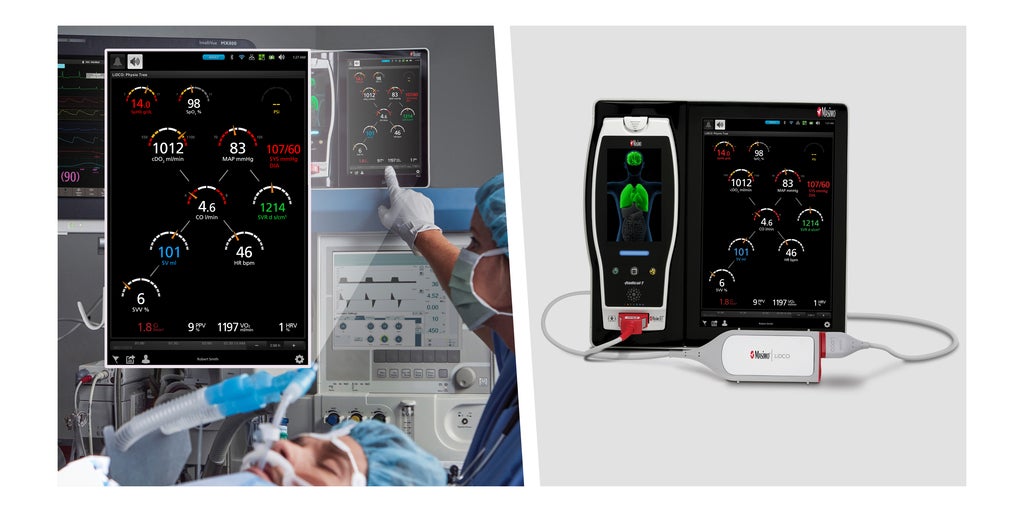
Medical technologies company Masimo has just received the EU Medical Device Regulation (MDR) CE mark for its LiDCO board-in-cable (BIC) module designed to connect to multi-patient monitoring platforms and provide greater oxygen delivery insights.
The LiDCO BIC module integrates with multi-patient monitoring platforms such as the Masimo Root Patient Monitoring and Connectivity Hub. This allows clinicians to enhance their hemodynamic monitoring capabilities by using Masimo's LiDCO technology, known for its PulseCO algorithm and eliminates the need for a hemodynamic monitoring box.
While the LiDCO module has received the CE mark it has not yet been cleared by the US Food and Drug Administration (FDA) and is not available in America.
Joe Kiani, CEO of Masimo said: "Bringing LiDCO’s beat-to-beat advanced hemodynamic monitoring to Masimo Root opens up the possibility of providing a more complete, continuous picture of cardiac output (CO) and oxygen delivery (DO2). Currently, hemodynamic monitors can provide continuous analysis of blood pressure information but rely on intermittent data from other monitors for oxygenation – giving clinicians only half of the information."
According to Masimo clinical studies have shown the benefits of using LiDCO in reducing complications, costs, and mortalities. In one randomized, controlled trial of 743 patients undergoing major abdominal surgery, hemodynamic optimisation with LiDCO led to a 20% reduction in complications.
Dr. Max Jonas, Consultant in Intensive Care Medicine and Anaesthesia at Southampton General Hospital in the UK said: “In my opinion understanding the individualized physiology of a particular patient is paramount to targeted treatments. Assessing preload, contractility and afterload using the LiDCO algorithm underpins patient management and the enhanced recovery after surgery (ERAS) process.”
Calling the collaboration an extraordinary advancement for anaesthesiology and intensive care Dr. Daniela Chaló, Head of Anaesthesiology at Centro Hospitalar Baixo Vouga in Portugal, added: “The addition of LiDCO to the Root monitor – which also offers SpHb for haemoglobin monitoring and SedLine and O3 for brain monitoring – means that all pertinent information can be available on one sole monitor.”