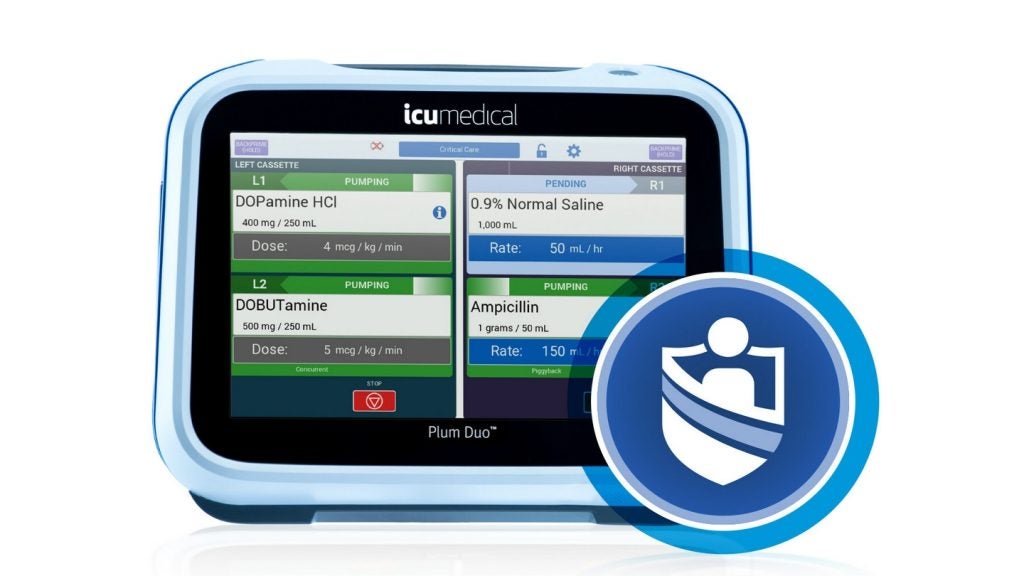
The US Food and Drug Administration (FDA) has granted 510(k) clearance for ICU Medical’s Plum Duo infusion pump with LifeShield infusion safety software.
Part of the company’s infusion device portfolio, the Plum Duo pump is based on the accurate delivery of Plum 360’s cassette technology.
Plum Duo comes with a large colour touchscreen with an advanced user interface and two channels that can deliver up to four compatible IV medications.
Designed as a unified cloud-based software suite, the LifeShield infusion safety software features advanced new tools to comprehensively manage the drug library.
It promotes collaboration by improving clinicians' capacity to readily access, process, and rapidly exchange information throughout an entire enterprise or health system.
The company plans to supply the Plum Duo pump and LifeShield software to US customers next year.
ICU Medical Infusion Systems general manager and corporate vice-president Dan Woolson said: “Receiving FDA clearance for the Plum Duo pump and LifeShield software is the first step in realising ICU Medical's long-term vision of bringing customers best-of-breed devices with a shared platform and user experience.
“We are proud of this milestone and look forward to bringing our next-generation Medfusion and CADD products to the LifeShield platform, creating the most complete, precise, and technologically advanced infusion systems in the industry for our customers and their patients.”
The company’s infusion system portfolio also includes Medfusion 4000 syringe pumps, CADD-Solispain management pumps, and CADD-Solis VIPambulatory pumps, as well as Plum 360 large volume pumps.