INBRAIN Neuroelectronics has secured breakthrough device designation (BDD) from the US Food and Drug Administration (FDA) for its Intelligent Network Modulation System as an adjunctive therapy to treat Parkinson’s disease.
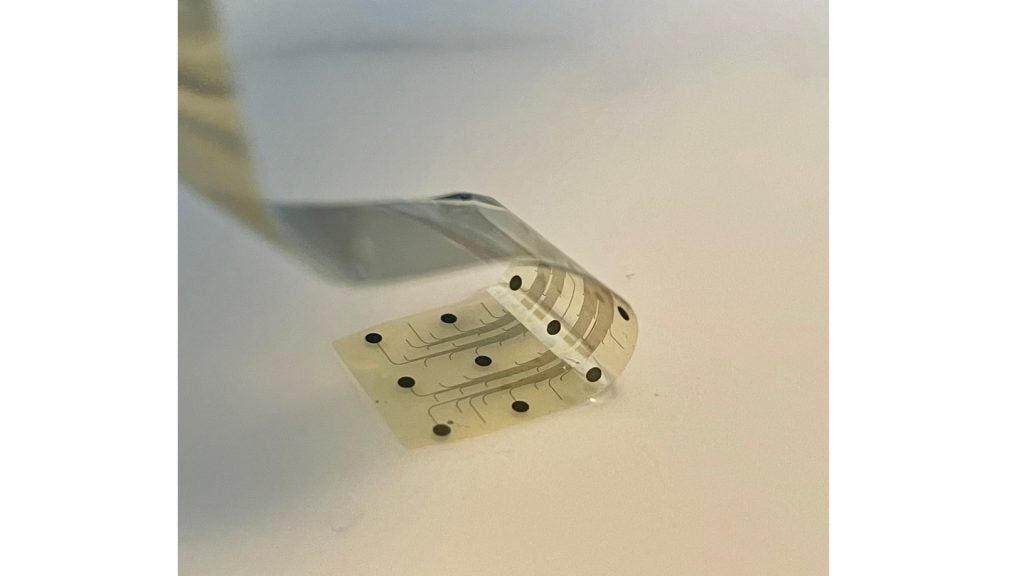
The INBRAIN system uses graphene, a two-dimensional material consisting of a lattice of carbon atoms and only one atom thick.
Graphene is claimed to be the thinnest known material and has a unique combination of electrical and mechanical properties suitable for neurotechnology innovation.
INBRAIN Neuroelectronics clinical affairs head Dan Gnansia said: “BDD from the FDA signifies the potential of the INBRAIN neural platform to further improve the lives of patients with Parkinson’s disease.
“We look forward to working with the agency to help bring this important advance into clinical practice."
INBRAIN’s neural platform technology facilitates ultra-high signal resolution.
It leverages machine learning software that decodes therapy-specific biomarkers for delivering adaptive neuroelectronic therapy to rebalance pathological neural networks.
INBRAIN Neuroelectronics CEO and co-founder Carolina Aguilar said: “INBRAIN is dedicated to leveraging new discoveries in materials science and transforming them into safe and effective breakthrough therapy applications.
“We anticipate developing our technology to treat other conditions affecting the central and peripheral nervous systems to make BCI technology relevant in neuro and bioelectronics.”
The company is focused on the development of the world’s first intelligent, graphene-neural platform for treating several disease conditions.
It develops semiconductor-based brain-computer interface technology for neuromodulation.
This technology will decode and modulate the activity of the brain in high resolution, utilising artificial intelligence to trigger personalised adaptive responses to treat conditions such as Parkinson's disease, epilepsy and speech impairment.