Johnson & Johnson (J&J) MedTech’s Shockwave Medical has launched the E⁸ peripheral intravascular (IVL) catheter in the US for patients living with certain types of peripheral artery disease (PAD).
Shockwave’s newest catheter, which is designed to deliver four hundred pulses twice per second and has eight emitters, is indicated for patients with calcified femoropopliteal and below-the-knee PAD, including patients with complex chronic limb-threatening ischemia (CLTI), the most severe form of the disease.
More than eight million people aged 40 years and older have PAD in the US, with two million of these patients living with CLTI, with the latter accounting for a large share of amputations occurring in the US each year.
The launch follows the US Food and Drug Administration (FDA) clearance of the technology in March this year, according to the agency’s 510(k) letter.
Shockwave was brought under the J&J MedTech umbrella earlier this year in a $13.1bn deal – one of the largest medtech acquisitions in recent years.
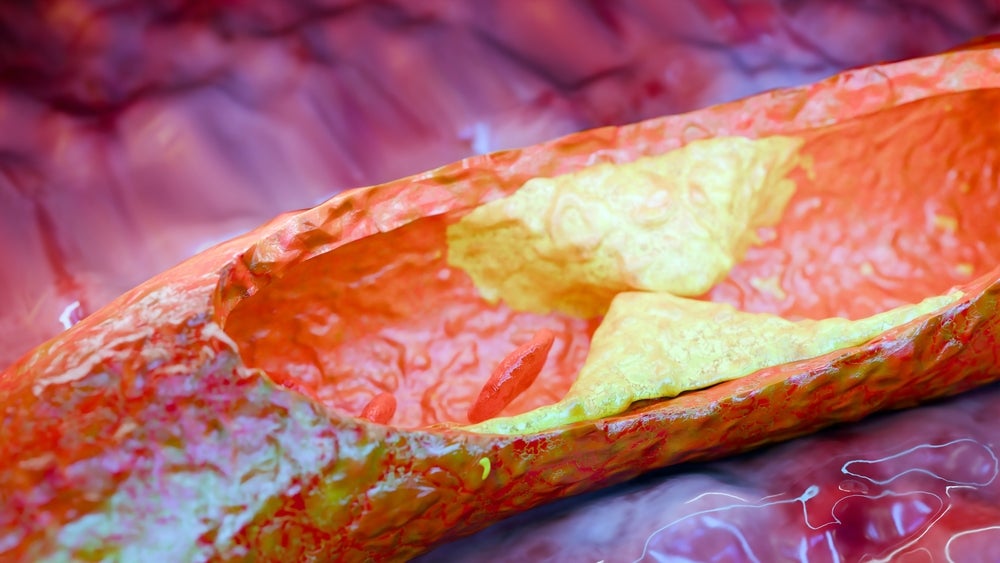
Alongside Shockwave’s PAD products, it also specialises in coronary artery disease (CAD). Core products for the company in these two areas centre around its IVL technology.
The balloon-mediated technology uses sonic pressure waves to crack calcium, the hallmark of plaque build-up, within the vessel wall.
Historically, angioplasty or stenting procedures were the first line of treatment for plaque removal in arteries. However, IVL catheters have since demonstrated superiority over standard angioplasty interventions.
According to GlobalData medical analyst Joselia Carlos, shockwave's IVL products immediately dominated the peripheral and coronary angioplasty market upon its entry in 2018 and 2021, respectively.
“Since then, Shockwave Medical has gained 32% of shares in the peripheral angioplasty market and 58% in the coronary angioplasty market within just four to five years of being in the space,” Carlos added when speaking to Medical Device Network at the time of the company’s acquisition by J&J.
The E⁸ catheter, which joins Shockwave’s L6, M5+, and S4 IVL catheters, has an increased working length of 150cm – allowing treatment to extend to distal lesions.
Shockwave’s president Isaac Zacharias said: “We have received very positive physician feedback on Shockwave E⁸ and look forward to establishing it as our new workhorse peripheral IVL catheter for physicians addressing challenging calcium above and below the knee.”