Jupiter Endovascular has treated the first two patients in the SPIRARE I study, utilising its Vertex pulmonary embolectomy (PE) system.
The trial helps in the development of endovascular procedures using the company's Endoportal Control platform technology.
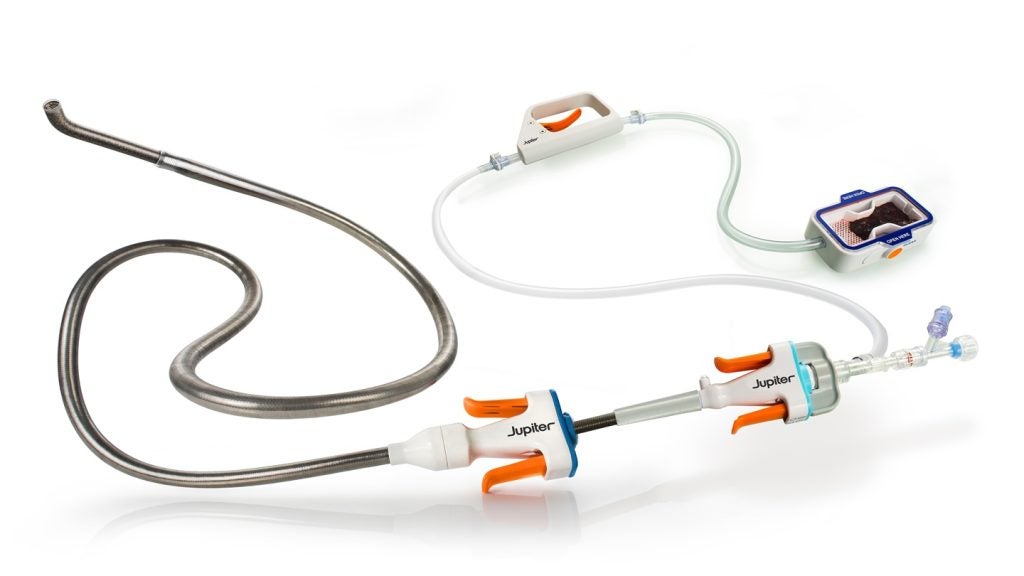
The Vertex system, designed for acute PE treatment, offers a new level of control and precision in endovascular procedures.
The first procedures were conducted at St John Paul II Hospital in Krakow, Poland, by a team of specialists, including professors Dr Grzysztof Bartus, Dr Grzegorz Kopec, and Dr Jakub Stepniewski.
SPIRARE I is a prospective, multicentre, single-arm study that is designed to enrol patients with acute, intermediate-risk PE to treat with the Vertex system. The study is set to expand to the Medical University of Vienna, where professor Irene Lang will lead as the principal investigator.
The study's endpoints will assess the procedural and clinical benefits of the Vertex system, focusing on safety, right heart function, and clinical improvement from the time of the procedure to 30 days after the procedure.
Jupiter Endovascular CEO Carl Bernard said: “As the first step in our SPIRARE clinical programme, we look forward to validating these positive results more broadly in this study and the upcoming SPIRARE II pivotal trial, as well as to expanding the potential benefits of our Endoportal Control platform technology to additional clinical areas where we intend to improve the lives and well-being of many patients.”
The Endoportal Control technology aims to enhance stability during catheter interventions, potentially treating sites that are challenging to reach with conventional methods.
The endoportal device, a key component of the Vertex system, is delivered in a flexible state over a guidewire, pressurised with saline to achieve a stable position for therapy delivery, and then relaxed for navigation or removal.
Last month, the US Food and Drug Administration granted approval for an investigational device exemption for the SPIRARE II US pivotal study to evaluate the system in treating acute PE.