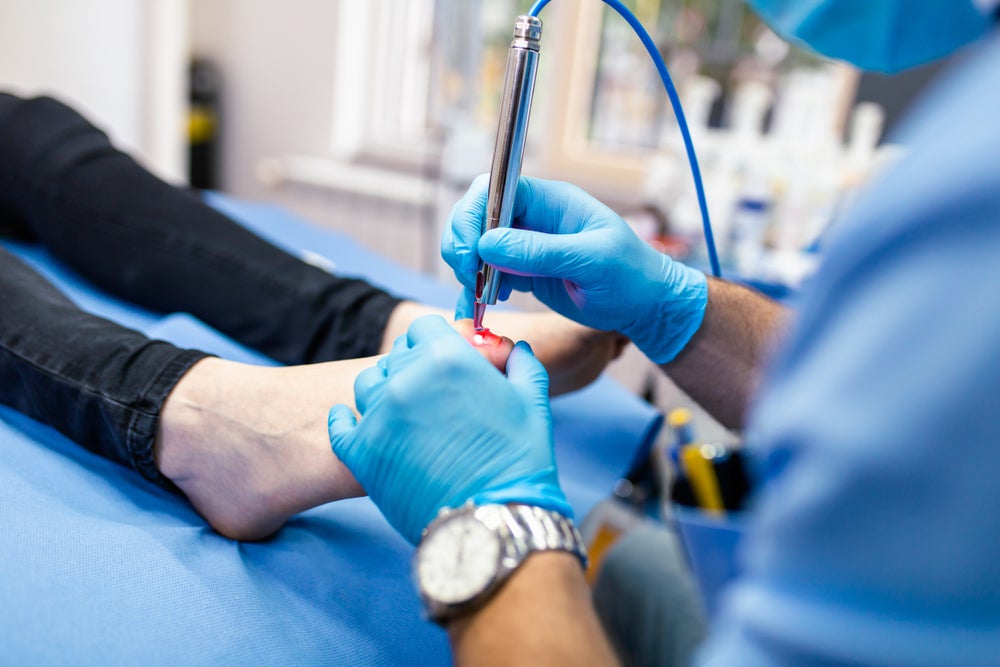
Scottish medtech company Emblation has kicked off its trial in Canada investigating the use of its Swift microwave skin therapy device for the treatment of onychomycosis, otherwise known as toenail fungus.
The company has recruited University of Toronto professor and MediProbe Research principal investigator Dr Aditya K Gupta to lead the clinical trial, which will see 45 patients undergo 12 months of treatment each.
The trial (NCT05674747) being conducted by MediProbe Research with Emblation as a collaborator, will have three groups of patients with different treatment plans.
One group will receive treatment weekly in the first month, then monthly for four months, for a total of nine treatments. A second group will receive treatments every two weeks in the first month, followed by four monthly treatments, for a total of seven treatments. The final group will receive treatment fortnightly for six months, having a total of 12 treatments.
The primary endpoints for the trial include the number of patients to complete the treatment regimen and the proportion of patients who demonstrate a temporary increase in clear nails.
Fungal nail infections are extremely common, with the US Centers for Disease Control and Prevention (CDC) estimating that around 14% of Americans are affected.
Current go-to treatments mostly revolve around antifungal creams. Emblation says that there are concerns that some strains of fungus are developing resistance to currently used drugs.
However, while new technology emerges to treat toenail infections such as employing the use of laser, this may not be a long-term cure as patients are only followed for a few months, according to NHS England. The trial with the Swift device, which has already been involved in the successful application for wart treatment, will monitor patients for a year.
Gupta said: “This is a condition with a large unmet medical need. We welcome Emblation in supporting our pilot investigational study as these patients presently have few effective treatment options."