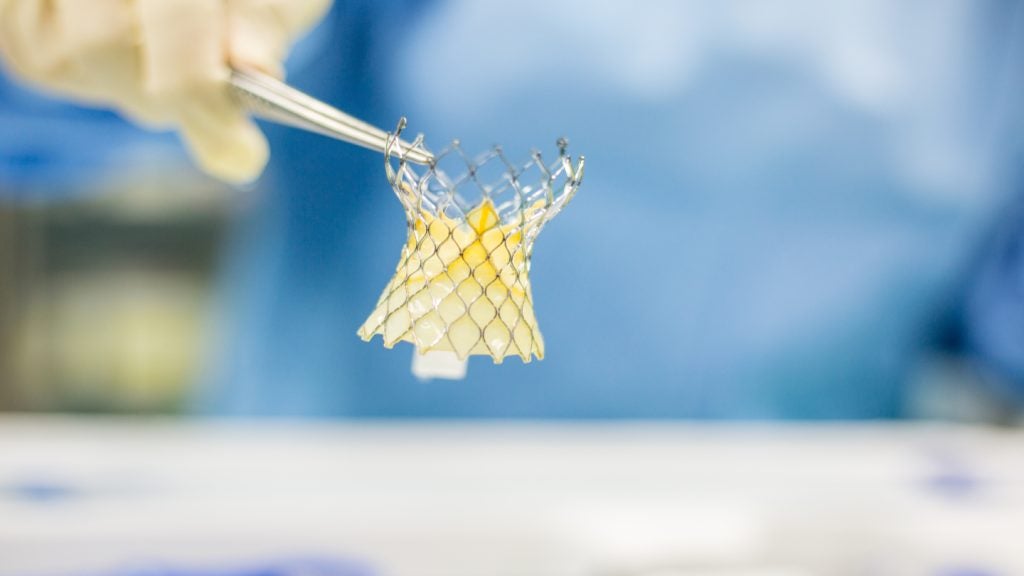
The US Food and Drug Administration (FDA) has granted approval to Medtronic the its Evolut FX+ transcatheter aortic valve replacement (TAVR) system.
The device is designed for the treatment of symptomatic severe aortic stenosis.
According to Medtronic, the Evolut FX+ system can offer increased coronary access windows through a modified diamond-shaped frame design, four times larger than previous designs.
It is the latest in Medtronic’s long-running TAVR range, a market in which a GlobalData market model estimates that Medtronic constitutes 33% of the US market at an estimated value of $561m. Sitting at second place behind Edwards Lifesciences 60.5% of the US market.
The total global market for TAVR devices is expected to be worth $6.8bn in 2023 and reach $18.8bn in 2033.
Severe aortic stenosis occurs when the aortic valve leaflets become stiff and have difficulty opening and closing, making the heart work harder to pump blood to the rest of the body.
Jeffrey Popma, chief medical officer for Medtronic’s structural heart and aortic business, said: “We are committed to consistently developing and advancing minimally invasive solutions for physicians to treat their patients with aortic stenosis.
“This is reinforced by our continued innovation of the Evolut TAVR platform, which has delivered proven valve performance and durability to physicians and patients for years. The Evolut FX+ TAVR system was designed to facilitate coronary access across a diverse range of patient anatomies with no compromise to valve performance.”
Besides TAVR devices, Medtronic is also a key player in the market of aortic guidewires where they are competing with US medtech company Boston Scientific. A market that GlobalData analysis predicts could become a key source of income as the rate of aortic stenosis procedures rises.
Elsewhere in the TAVR system market, Edwards Lifesciences has revealed positive data from two real-world studies evaluating its SAPIEN valve platform, finding good all-cause mortality and stroke outcomes in patients. At the same time, Germany-based Protembis has raised $30m in a Series B funding round to initiate a pivotal clinical trial of its intra-aortic filter device.