Terumo subsidiary and neurovascular company MicroVention has announced the commercial availability of the LVIS EVO Intraluminal Support Device in the US.
Available in Europe since 2019, the device is designed for the treatment of wide-neck intracranial aneurysms.
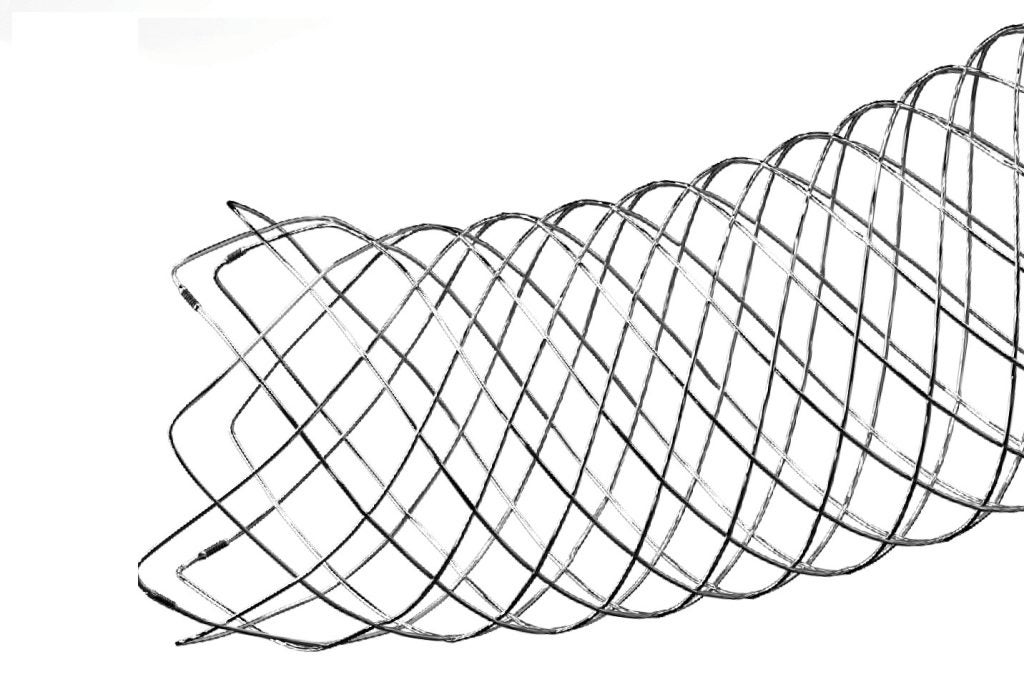
Said to be the first fully visible coil-assist-intracranial stent in the US, the LVIS EVO is equipped to offer enhanced visualisation, allowing for wall apposition confirmation, and is fully visible under fluoroscopy.
The stent's advanced braid design and DFT wire construction contribute to an optimised opening and precise placement.
The device's braid angle has been optimised to improve device opening along its entire length.
Additionally, the device provides controlled delivery and development, with the capability to resheath up to 80% of the device's length, ensuring precise placement.
Since its European launch, more than 12,000 LVIS EVO units have been sold.
In the US, the device is indicated for use with neurovascular embolisation coils in patients aged 18 and above for the treatment of wide-neck saccular intracranial aneurysms.
MicroVention president and CEO Carsten Schroeder said: “Today’s announcement underscores MicroVention’s commitment to delivering groundbreaking solutions for haemorrhagic stroke treatment.
“MicroVention continues to lead the way in both hemorrhagic and ischemic stroke care. This achievement is a testament to our collaboration with leading physicians worldwide. By identifying the evolving needs in patient care and transforming those insights into innovative technologies, we are able to save lives and improve outcomes.”
MicroVention said its LVIS EVO portfolio is compatible with its Headway 17 Advanced Microcatheter and Scepter C and Scepter XC Occlusion Balloons.
Last year, MicroVention launched the ERIC Retrieval Device in the US for ischemic stroke treatment.