Natus Medical has sought US Food and Drug Administration (FDA) clearance for its point-of-care EEG device.
The company submitted a 510(k) premarket notification to the FDA in this regard.
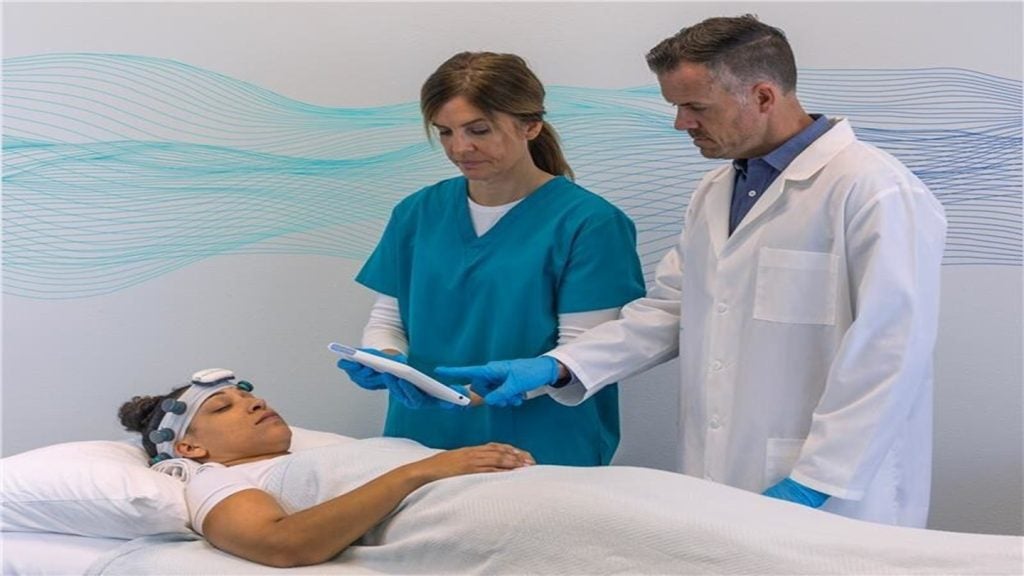
This decision-support tool is engineered to rapidly identify non-convulsive seizures and status epilepticus in acute care settings, thereby facilitating swift intervention and enhancing patient outcomes.
It utilises Natus' NeuroWorks software, a platform that offers a consistent review experience and enables remote collaboration with neurologists.
Natus has established a partnership with Persyst to integrate seizure detection algorithms powered by AI, which are also currently under FDA 510(k) review.
Healthcare professionals who have evaluated the system have high expectations for the EEG device from Natus, which provides EEG neurodiagnostic solutions.
The feedback indicates that the system is user-friendly and can be set up quickly by emergency room and ICU staff without the need for a specialised EEG technologist, while also delivering clinical information that meets neurologists' expectations.
The device is expected to offer the precision and confidence that healthcare providers require to make critical decisions in emergency and intensive care situations.
Natus will use a cloud platform certified by cybersecurity to ensure security of patient data.
The company provides solutions for screening, diagnosing and treating neurological disorders. Its comprehensive solutions including services, field support and education empower clinicians to elevate their standard of care, thereby improving patient outcomes.
Natus also offers other neuro solutions such as electromyography, polysomnography, as well as neurocritical and brain injury solutions.