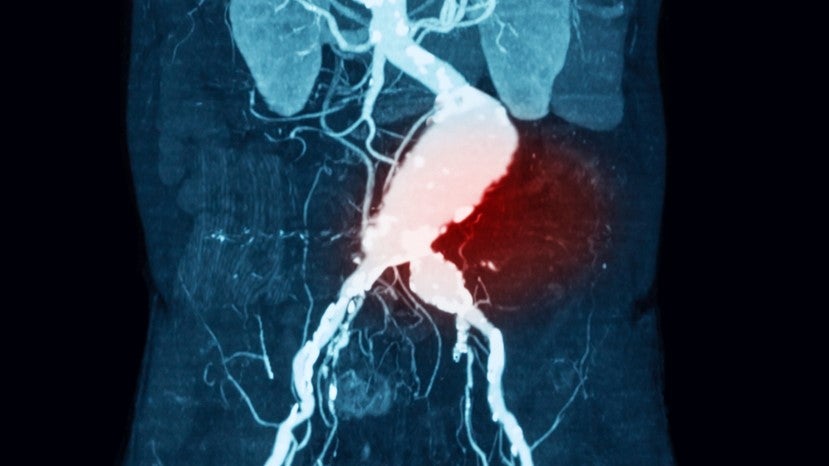
Arizona-based Nectro Medical has initiated a Phase II/III trial evaluating the safety and efficacy of its endovascular aneurysm stabilisation treatment (Nectero EAST).
Nectero EAST is a single-use, endovascular system made up of a dual-balloon delivery catheter and stabiliser mixture of pentagalloyl glucose (PGG). The PGG, when administered locally, can bind to elastin and collagen to potentially strengthen the aortic wall. Animal studies have shown that PGG can hinder the development of abdominal aortic aneurysm (AAA).
TheT device received a fast-track designation from the US Food and Drug Administration (FDA) in August 2023 and theprocedure can be completed in an hour without the need for any specialised tools.
Nectero EAST clinical trial
The Phase II/III stAAAble trial (NCT06001918) will investigate the safety and efficacy of the Nectero EAST device in patients with infrarenal AAAs, with a maximum diameter of 3.5–5 cm. The trial is expected to enrol 400 patients across multiple sites in the US andwill compare Nectero EAST with the current standard of care - surveillance.
The primary endpoint of the trial is the number of participants experiencing AAA-related death, rupture, and repair during the 24 months. The trial’s secondary endpoint is growth in aneurysm diameter over time based on CT scan core laboratory readings at 24 months.
Following the study’s conclusion at 24 months, the patients will receive annual follow-ups till the fifth year since treatment.
The results from the 11 patients who received follow-up for 3 years in the first human trial (NCT05133492) of the Nectero EAST device were published in the Journal of Vascular Surgery. The average changes in maximum aneurysm diameter from baseline to 6, 12, 24, and 36 months were 0.2 mm, 1.1 mm, 1.2 mm, and 0.8 mm, respectively.
The cardiovascular device market is expected to grow from being worth approximately $59.9bn in 2023 to over $86.6bn in 2030, as per GlobalData market analysis.