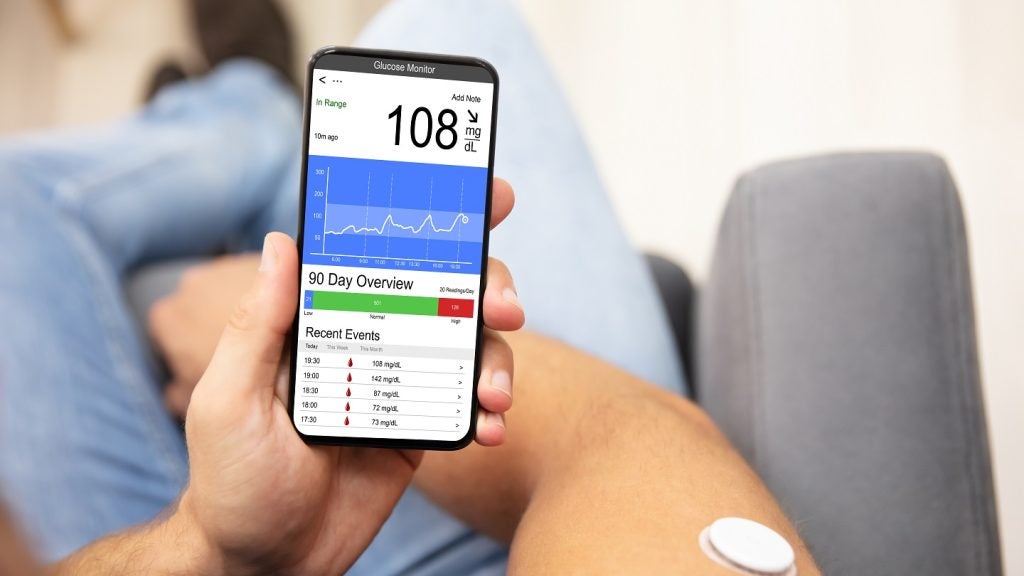
Nemaura Medical has completed a 100-patient study of the non-invasive and flexible continuous glucose monitor sugarBEAT.
Carried out in the Middle East, the study included four cohorts with an equal number of male and female subjects aged 18 to 75 years.
Out of the total patients, 20 had type 1 diabetes and the remaining had type 2 diabetes.
The Class IIb medical device is approved in Europe and Saudi Arabia for a wear period of 14 hours.
The study was designed to evaluate the possibility of increasing the wear period to up to 24 hours and assess different methods of device application to the skin, as well as the possibility of auto-calibration.
The interim data of the first cohort, including 25 patients on single-day sensor wear, over a 12-hour period demonstrated an overall mean absolute relative difference (MARD) of 9.8%, with 86% of the paired points within 20/20 of the reference value.
An overall MARD of 12.8% was observed over a 24-hour sensor wear period, based on 1,379 paired points, with 76% of the paired points within 20/20 of the reference blood serum glucose value.
These results indicate the possibility of using a single sensor for a 24-hour sensor wear period, allowing users to monitor their glucose fluctuations overnight.
The remaining cohorts are being evaluated to establish the extent to which auto-calibration may be feasible and to determine the optimal sensor application method.
The company’s other non-invasive wearable diagnostic devices include proBEAT.