Neurotechnology developer Neurolief has secured a strategic equity investment from Japan’s Sawai Group.
The investment will strengthen the partnership between Neurolief and Sawai, following an exclusive agreement signed by the companies for the development and marketing of Sawai's therapy Relivion in Japan.
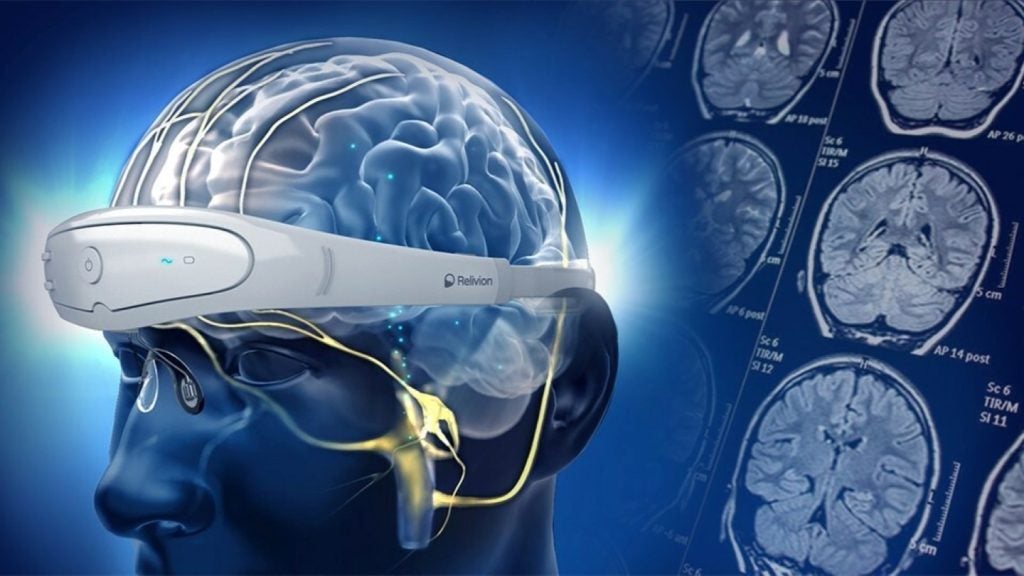
Relivion is a new, non-invasive, at-home wearable neuromodulation therapy designed for treating migraine and depression.
It stimulates six branches of the occipital and trigeminal nerves, which can trigger migraines.
The therapy has shown high clinical efficiency, which was only possible with invasive procedures.
Users can wear the device around the head and the electrodes will be accurately positioned above the target nerves for efficient stimulation. Its level of intensity can be adjusted for patient comfort.
The device recently secured approval in Japan for treating migraine, and an application for its use in treating depression is currently being prepared.
Neurolief CEO Scott Drees said: “We are very pleased to receive this investment from our strategic partner, Sawai Group Holdings.
“It not only underscores our shared vision for utilising Neurolief's disruptive technology to treat millions of patients suffering from migraine and depression in Japan but also represents a shared commitment to improving the care of these debilitating chronic diseases.”
Sawai Group CEO Mitsuo Sawai said: “Guided by our corporate philosophy of 'Always Putting Patients First,' we are dedicated to contributing to healthier lives by introducing new and innovative treatment options.
“With the introduction of Relivion for at-home treatment under doctor supervision, we are expanding treatment choices for patients suffering from either migraine or depression.”
Last July, Neurolief announced positive results from an interim analysis of a randomised controlled pivotal trial of Relivion to treat major depressive disorder (MDD).
The prospective, placebo-controlled, multi-centre, randomised, double-blind MOOD clinical trial was carried out in 12 US clinical sites and one in Israel.